Abstract
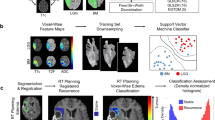
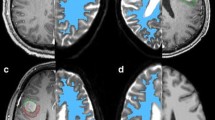
Conflicting results on differentiating edema and glioma by diffusion tensor imaging (DTI) are possibly attributable to dissimilar spatial distribution of the lesions. Combining DTI-parameters and enhanced registration might improve prediction. Regions of edema surrounding 22 metastases were compared to tumor-infiltrated regions from WHO grade 2 (12), 3 (10) and 4 (18) gliomas. DTI data was co-registered using Tract Based Spatial Statistics (TBSS), to measure Fractional Anisotropy (FA) and Mean Diffusivity (MD) for white matter only, and relative changes compared to matching reference regions (dFA and dMD). A two-factor principal component analysis (PCA) on metastasis and grade 2 glioma was performed to explore a possible differentiating combined factor. Edema demonstrated equal MD and higher FA compared to grade 2 and 3 glioma (P < 0.001), but did not differ from glioblastoma. Differences were non-significant when corrected for spatial distribution, since reference regions differed strongly (P < 0.001). The second component of the PCA (PCA-C2) did differentiate edema and low-grade tumor (sensitivity 91.7 %, specificity 86.4 %). PCA-C2 scores were plotted voxel-wise as a probability-map, discerning distinct areas of presumed edema or tumor infiltration. Correction of spatial dependency appears essential when differentiating glioma from edema. A tumor-infiltration probability-map is presented, based on supplementary information of multiple DTI parameters and spatial normalization.




Similar content being viewed by others
References
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401
Keles GE, Chang EF, Lamborn KR, Tihan T, Chang CJ, Chang SM et al (2006) Volumetric extent of resection and residual contrast enhancement on initial surgery as predictors of outcome in adult patients with hemispheric anaplastic astrocytoma. J Neurosurg 105:34–40
Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S et al (2008) Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 26:1338–1345
Jena R, Price SJ, Baker C, Jefferies SJ, Pickard JD, Gillard JH et al (2005) Diffusion tensor imaging: possible implications for radiotherapy treatment planning of patients with high-grade glioma. Clin Oncol (R Coll Radiol) 17(8):581–590
Hatakeyama T, Kawai N, Nishiyama Y, Yamamoto Y, Sasakawa Y, Ichikawa T et al (2008) 11C-methionine (MET) and 18F-fluorothymidine (FLT) PET in patients with newly diagnosed glioma. Eur J Nucl Med Mol Imaging 35:2009–2017
Pauleit D, Floeth F, Hamacher K, Riemenschneider MJ, Reifenberger G, Muller HW et al (2005) O-(2-[18F]fluoroethyl)-l-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain 128:678–687
Floeth FW, Pauleit D, Wittsack HJ, Langen KJ, Reifenberger G, Hamacher K et al (2005) Multimodal metabolic imaging of cerebral gliomas: positron emission tomography with [18F]fluoroethyl-l-tyrosine and magnetic resonance spectroscopy. J Neurosurg 102:318–327
Chiang IC, Kuo YT, Lu CY, Yeung KW, Lin WC, Sheu FO et al (2004) Distinction between high-grade gliomas and solitary metastases using peritumoral 3-T magnetic resonance spectroscopy, diffusion, and perfusion imagings. Neuroradiology 46:619–627
Law M, Cha S, Knopp EA, Johnson G, Arnett J, Litt AW (2002) High-grade gliomas and solitary metastases: differentiation by using perfusion and proton spectroscopic MR imaging. Radiology 222:715–721
Di Costanzo A, Scarabino T, Trojsi F, Giannatempo GM, Popolizio T, Catapano D et al (2006) Multiparametric 3T MR approach to the assessment of cerebral gliomas: tumor extent and malignancy. Neuroradiology 48:622–631
Bastin ME, Sinha S, Whittle IR, Wardlaw JM (2002) Measurements of water diffusion and T1 values in peritumoural oedematous brain. NeuroReport 13:1335–1340
Kinoshita M, Goto T, Okita Y, Kagawa N, Kishima H, Hashimoto N et al (2010) Diffusion tensor-based tumor infiltration index cannot discriminate vasogenic edema from tumor-infiltrated edema. J Neurooncol 96:409–415
Lu S, Ahn D, Johnson G, Cha S (2003) Peritumoral diffusion tensor imaging of high-grade gliomas and metastatic brain tumors. AJNR Am J Neuroradiol 24:937–941
Lu S, Ahn D, Johnson G, Law M, Zagzag D, Grossman RI (2004) Diffusion-tensor MR imaging of intracranial neoplasia and associated peritumoral edema: introduction of the tumor infiltration index. Radiology 232:221–228
Morita K, Matsuzawa H, Fujii Y, Tanaka R, Kwee IL, Nakada T (2005) Diffusion tensor analysis of peritumoral edema using lambda chart analysis indicative of the heterogeneity of the microstructure within edema. J Neurosurg 102:336–341
Oh J, Cha S, Aiken AH, Han ET, Crane JC, Stainsby JA et al (2005) Quantitative apparent diffusion coefficients and T2 relaxation times in characterizing contrast enhancing brain tumors and regions of peritumoral edema. J Magn Reson Imaging 21:701–708
Pauleit D, Langen KJ, Floeth F, Hautzel H, Riemenschneider MJ, Reifenberger G et al (2004) Can the apparent diffusion coefficient be used as a noninvasive parameter to distinguish tumor tissue from peritumoral tissue in cerebral gliomas? J Magn Reson Imaging 20:758–764
Price SJ, Jena R, Burnet NG, Hutchinson PJ, Dean AF, Pena A et al (2006) Improved delineation of glioma margins and regions of infiltration with the use of diffusion tensor imaging: an image-guided biopsy study. AJNR Am J Neuroradiol 27:1969–1974
Provenzale JM, McGraw P, Mhatre P, Guo AC, Delong D (2004) Peritumoral brain regions in gliomas and meningiomas: investigation with isotropic diffusion-weighted MR imaging and diffusion-tensor MR imaging. Radiology 232:451–460
Wang S, Kim S, Chawla S, Wolf RL, Zhang WG, O’Rourke DM et al (2009) Differentiation between glioblastomas and solitary brain metastases using diffusion tensor imaging. Neuroimage 44:653–660
Wang S, Kim S, Chawla S, Wolf RL, Knipp DE, Vossough A et al (2011) Differentiation between glioblastomas, solitary brain metastases, and primary cerebral lymphomas using diffusion tensor and dynamic susceptibility contrast-enhanced MR imaging. AJNR Am J Neuroradiol 32:507–514
Wang W, Steward CE, Desmond PM (2009) Diffusion tensor imaging in glioblastoma multiforme and brain metastases: the role of p, q, L, and fractional anisotropy. AJNR Am J Neuroradiol 30:203–208
Stadlbauer A, Ganslandt O, Buslei R, Hammen T, Gruber S, Moser E et al (2006) Gliomas: histopathologic evaluation of changes in directionality and magnitude of water diffusion at diffusion-tensor MR imaging. Radiology 240:803–810
Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL et al (2007) Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 36:630–644
Kang X, Herron TJ, Woods DL (2011) Regional variation, hemispheric asymmetries and gender differences in pericortical white matter. Neuroimage 56:2011–2023
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE et al (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31:1487–1505
Inano S, Takao H, Hayashi N, Abe O, Ohtomo K (2011) Effects of age and gender on white matter integrity. AJNR Am J Neuroradiol 32(11):2103–2109
Abe O, Takao H, Gonoi W, Sasaki H, Murakami M, Kabasawa H et al (2010) Voxel-based analysis of the diffusion tensor. Neuroradiology 52:699–710
Pavlisa G, Rados M, Pavlisa G, Pavic L, Potocki K, Mayer D (2009) The differences of water diffusion between brain tissue infiltrated by tumor and peritumoral vasogenic edema. Clin Imaging 33:96–101
Min ZG, Niu C, Rana N, Ji HM, Zhang M (2013) Differentiation of pure vasogenic edema and tumor-infiltrated edema in patients with peritumoral edema by analyzing the relationship of axial and radial diffusivities on 3.0T MRI. Clin Neurol Neurosurg 115:1366–1370
Murakami R, Hirai T, Sugahara T, Fukuoka H, Toya R, Nishimura S et al (2009) Grading astrocytic tumors by using apparent diffusion coefficient parameters: superiority of a one- versus two-parameter pilot method. Radiology 251:838–845
Pope WB, Kim HJ, Huo J, Alger J, Brown MS, Gjertson D et al (2009) Recurrent glioblastoma multiforme: a DC histogram analysis predicts response to bevacizumab treatment. Radiology 252:182–189
Chu HH, Choi SH, Ryoo I, Kim SC, Yeom JA, Shin H et al (2013) Differentiation of true progression from pseudoprogression in glioblastoma treated with radiation therapy and concomitant temozolomide: comparison study of standard and high-b-value diffusion-weighted imaging. Radiology 269:831–840
Barajas RF Jr, Phillips JJ, Parvataneni R, Molinaro A, Essock-Burns E, Bourne G et al (2012) Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR Imaging. Neuro Oncol 14:942–954
Price SJ, Pena A, Burnet NG, Jena R, Green HA, Carpenter TA et al (2004) Tissue signature characterisation of diffusion tensor abnormalities in cerebral gliomas. Eur Radiol 14:1909–1917
Cortez-Conradis D, Favila R, Isaac-Olive K, Martinez-Lopez M, Rios C, Roldan-Valadez E (2013) Diagnostic performance of regional DTI-derived tensor metrics in glioblastoma multiforme: simultaneous evaluation of p, q, L, Cl, Cp, Cs, RA, RD, AD, mean diffusivity and fractional anisotropy. Eur Radiol 23:1112–1121
Ellingson BM, Kim HJ, Woodworth DC, Pope WB, Cloughesy JN, Harris RJ et al (2013) Recurrent glioblastoma treated with Bevacizumab: contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a Multicenter Clinical Trial. Radiology 271(1):200–210
Beppu T, Inoue T, Shibata Y, Yamada N, Kurose A, Ogasawara K et al (2005) Fractional anisotropy value by diffusion tensor magnetic resonance imaging as a predictor of cell density and proliferation activity of glioblastomas. Surg Neurol 63:56–61
Server A, Graff BA, Orheim TE, Schellhorn T, Josefsen R, Gadmar OB et al (2011) Measurements of diagnostic examination performance and correlation analysis using microvascular leakage, cerebral blood volume, and blood flow derived from 3T dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging in glial tumor grading. Neuroradiology 53:435–447
Roy B, Gupta RK, Maudsley AA, Awasthi R, Sheriff S, Gu M et al (2013) Utility of multiparametric 3-T MRI for glioma characterization. Neuroradiology 55:603–613
Chung WJ, Kim HS, Kim N, Choi CG, Kim SJ (2013) Recurrent glioblastoma: optimum area under the curve method derived from dynamic contrast-enhanced T1-weighted perfusion MR imaging. Radiology 269:561–568
Law M, Young RJ, Babb JS, Peccerelli N, Chheang S, Gruber ML et al (2008) Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 247:490–498
Server A, Orheim TE, Graff BA, Josefsen R, Kumar T, Nakstad PH (2011) Diagnostic examination performance by using microvascular leakage, cerebral blood volume, and blood flow derived from 3-T dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging in the differentiation of glioblastoma multiforme and brain metastasis. Neuroradiology 53:319–330
Pirotte B, Goldman S, Massager N, David P, Wikler D, Vandesteene A et al (2004) Comparison of 18F-FDG and 11C-methionine for PET-guided stereotactic brain biopsy of gliomas. J Nucl Med 45:1293–1298
Price SJ, Fryer TD, Cleij MC, Dean AF, Joseph J, Salvador R et al (2009) Imaging regional variation of cellular proliferation in gliomas using 3′-deoxy-3′-[18F]fluorothymidine positron-emission tomography: an image-guided biopsy study. Clin Radiol 64:52–63
Floeth FW, Sabel M, Ewelt C, Stummer W, Felsberg J, Reifenberger G et al (2011) Comparison of (18)F-FET PET and 5-ALA fluorescence in cerebral gliomas. Eur J Nucl Med Mol Imaging 38:731–741
Kracht LW, Miletic H, Busch S, Jacobs AH, Voges J, Hoevels M et al (2004) Delineation of brain tumor extent with [11C]l-methionine positron emission tomography: local comparison with stereotactic histopathology. Clin Cancer Res 10:7163–7170
Kinoshita M, Arita H, Goto T, Okita Y, Isohashi K, Watabe T et al (2012) A novel PET index, 18F-FDG-11C-methionine uptake decoupling score, reflects glioma cell infiltration. J Nucl Med 53:1701–1708
McKnight TR, dem Bussche MH, Vigneron DB, Lu Y, Berger MS, McDermott MW et al (2002) Histopathological validation of a three-dimensional magnetic resonance spectroscopy index as a predictor of tumor presence. J Neurosurg 97:794–802
Guo J, Yao C, Chen H, Zhuang D, Tang W, Ren G et al (2012) The relationship between Cho/NAA and glioma metabolism: implementation for margin delineation of cerebral gliomas. Acta Neurochir 154:1361–1370
Server A, Josefsen R, Kulle B, Maehlen J, Schellhorn T, Gadmar O et al (2010) Proton magnetic resonance spectroscopy in the distinction of high-grade cerebral gliomas from single metastatic brain tumors. Acta Radiol 51:316–325
Damoiseaux JS, Smith SM, Witter MP, Sanz-Arigita EJ, Barkhof F, Scheltens P et al (2009) White matter tract integrity in aging and Alzheimer’s disease. Hum Brain Mapp 30:1051–1059
Kern KC, Sarcona J, Montag M, Giesser BS, Sicotte NL (2011) Corpus callosal diffusivity predicts motor impairment in relapsing-remitting multiple sclerosis: a TBSS and tractography study. Neuroimage 55:1169–1177
Focke NK, Yogarajah M, Bonelli SB, Bartlett PA, Symms MR, Duncan JS (2008) Voxel-based diffusion tensor imaging in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Neuroimage 40:728–737
Weinstein M, Ben-Sira L, Levy Y, Zachor DA, Itzhak EB, Artzi M et al (2011) Abnormal white matter integrity in young children with autism. Hum Brain Mapp 32:534–543
Ethical Standards
The current work complies with the current laws of the country in which they were performed (The Netherlands).
Conflict of interest
The authors declare that they have no actual or potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hoefnagels, F.W.A., De Witt Hamer, P., Sanz-Arigita, E. et al. Differentiation of edema and glioma infiltration: proposal of a DTI-based probability map. J Neurooncol 120, 187–198 (2014). https://doi.org/10.1007/s11060-014-1544-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1544-9