Abstract
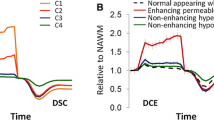
Differentiation between treatment-related changes and progressive disease (PD) remains a major clinical challenge in the follow-up of patients with high grade brain tumors. The aim of this study was to differentiate between treatment-related changes and PD using dynamic contrast enhanced (DCE) MRI. Twenty patients were scanned using conventional, DCE-MRI and MR spectroscopy (total of 44 MR scans). The enhanced lesion area was extracted using independent components analysis of the DCE data. Pharmacokinetic parameters were estimated from the DCE data based on the Extended-Tofts-Model. Voxel based classification for treatment-related changes versus PD was performed in a patient-wise leave-one-out manner, using a support vector machine classifier. DCE parameters, K trans, v e, k ep and v p, significantly differentiated between the tissue types. Classification results were validated using spectroscopy data showing significantly higher choline/creatine values in the extracted PD component compared to areas with treatment-related changes and normal appearing white matter, and high correlation between choline/creatine values and the percentage of the identified PD component within the lesion area (r = 0.77, p < 0.001). On the training data the sensitivity and specificity were 98 and 97 %, respectively, for the treatment-related changes component and 97 and 98 % for the PD component. This study proposes a methodology based on DCE-MRI to differentiate lesion areas into treatment-related changes versus PD, prospectively in each scan. Results may have major clinical importance for pre-operative planning, guidance for targeting biopsy, and early prediction of radiological outcomes in patients with high grade brain tumors.




Similar content being viewed by others
References
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. doi:10.1200/JCO.2009.26.3541
Ruben JD, Dally M, Bailey M, Smith R, McLean CA, Fedele P (2006) Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys 65:499–508. doi:10.1016/j.ijrobp.2005.12.002
Kumar AJ, Leeds NE, Fuller GN, Van Tassel P, Maor MH, Sawaya RE, Levin VA (2000) Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 217:377–384. doi:10.1148/radiology.217.2.r00nv36377
Ulmer S, Braga TA, Barker FG 2nd, Lev MH, Gonzalez RG, Henson JW (2006) Clinical and radiographic features of peritumoral infarction following resection of glioblastoma. Neurology 67:1668–1670. doi:10.1212/01.wnl.0000242894.21705.3c
Wong CS, Van der Kogel AJ (2004) Mechanisms of radiation injury to the central nervous system: implications for neuroprotection. Mol Interv 4:273–284. doi:10.1124/mi.4.5.7
Lyubimova N, Hopewell JW (2004) Experimental evidence to support the hypothesis that damage to vascular endothelium plays the primary role in the development of late radiation-induced CNS injury. Br J Radiol 77:488–492
Verma N, Cowperthwaite MC, Burnett MG, Markey MK (2013) Differentiating tumor recurrence from treatment necrosis: a review of neuro-oncologic imaging strategies. Neuro Oncol 15:515–534. doi:10.1093/neuonc/nos307
Brandes AA, Tosoni A, Spagnolli F, Frezza G, Leonardi M, Calbucci F, Franceschi E (2008) Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro Oncol 10:361–367. doi:10.1215/15228517-2008-008
Pope WB, Young JR, Ellingson BM (2011) Advances in MRI assessment of gliomas and response to anti-VEGF therapy. Curr Neurol Neurosci Rep 11:336–344. doi:10.1007/s11910-011-0179-x
Barajas RF Jr, Chang JS, Segal MR, Parsa AT, McDermott MW, Berger MS, Cha S (2009) Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 253:486–496. doi:10.1148/radiol.2532090007
Hu LS, Baxter LC, Smith KA, Feuerstein BG, Karis JP, Eschbacher JM, Coons SW, Nakaji P, Yeh RF, Debbins J, Heiserman JE (2009) Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol 30:552–558. doi:10.3174/ajnr.A1377
Bobek-Billewicz B, Stasik-Pres G, Majchrzak H, Zarudzki L (2010) Differentiation between brain tumor recurrence and radiation injury using perfusion, diffusion-weighted imaging and MR spectroscopy. Folia Neuropathol 48:81–92
Kim J, Leira EC, Callison RC, Ludwig B, Moritani T, Magnotta VA, Madsen MT (2010) Toward fully automated processing of dynamic susceptibility contrast perfusion MRI for acute ischemic cerebral stroke. Comput Methods Programs Biomed 98:204–213
Barajas RF, Chang JS, Sneed PK, Segal MR, McDermott MW, Cha S (2009) Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol 30:367–372. doi:10.3174/ajnr.A1362
Sourbron SP, Buckley DL (2013) Classic models for dynamic contrast-enhanced MRI. NMR Biomed 26:1004–1027. doi:10.1002/nbm.2940
Tofts PS, Kermode AG (1991) Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med 17:357–367
Liberman G, Nadav G, Louzoun Y, Artzi M, Ben Bashat D (2014) Bolus arrival time extraction using super temporal resolution analysis of DCE. International Society for Magnetic Resonance in Medicine, Milan
Bisdas S, Naegele T, Ritz R, Dimostheni A, Pfannenberg C, Reimold M, Koh TS, Ernemann U (2011) Distinguishing recurrent high-grade gliomas from radiation injury: a pilot study using dynamic contrast-enhanced MR imaging. Acad Radiol 18:575–583. doi:10.1016/j.acra.2011.01.018
Larsen VA, Simonsen HJ, Law I, Larsson HB, Hansen AE (2013) Evaluation of dynamic contrast-enhanced T1-weighted perfusion MRI in the differentiation of tumor recurrence from radiation necrosis. Neuroradiology 55:361–369. doi:10.1007/s00234-012-1127-4
Narang J, Jain R, Arbab AS, Mikkelsen T, Scarpace L, Rosenblum ML, Hearshen D, Babajani-Feremi A (2011) Differentiating treatment-induced necrosis from recurrent/progressive brain tumor using nonmodel-based semiquantitative indices derived from dynamic contrast-enhanced T1-weighted MR perfusion. Neuro Oncol 13:1037–1046. doi:10.1093/neuonc/nor075
Deoni SC, Peters TM, Rutt BK (2004) High-resolution T1 and T2 mapping of the brain in a clinically acceptable time with DESPOT1 and DESPOT2. Magn Reson Med 53:237–241
Liberman G, Louzoun Y, Ben Bashat D (2013) T1 mapping using variable flip angle SPGR data with flip angle correction. J Magn Reson Imaging 40:171–180
Jenkinson M, Smith S (2001) A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156
Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. doi:10.1002/hbm.10062
Sled JG, Zijdenbos AP, Evans AC (1998) A nonparametric method for automatic correction of intensity nonuniformity in MRI data. Med Imaging IEEE Trans 17:87–97
Deoni SC, Peters TM, Rutt BK (2005) High-resolution T1 and T2 mapping of the brain in a clinically acceptable time with DESPOT1 and DESPOT2. Magn Reson Med 53:237–241
Murase K (2004) Efficient method for calculating kinetic parameters using T1-weighted dynamic contrast-enhanced magnetic resonance imaging. Magn Reson Med 51:858–862. doi:10.1002/mrm.20022
Bagher-Ebadian H, Jain R, Nejad-Davarani SP, Mikkelsen T, Lu M, Jiang Q, Scarpace L, Arbab AS, Narang J, Soltanian-Zadeh H, Paudyal R, Ewing JR (2012) Model selection for DCE-T1 studies in glioblastoma. Magn Reson Med 68:241–251. doi:10.1002/mrm.23211
Beckmann CF, Smith SM (2004) Probabilistic independent component analysis for functional magnetic resonance imaging. Med Imaging IEEE Trans 23:137–152
Provencher SW (1993) Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30:672–679
Hopewell JW, Calvo W, Jaenke R, Reinhold HS, Robbins ME, Whitehouse EM (1993) Microvasculature and radiation damage. Recent Results Cancer Res 130:1–16
Mehrabian H, Chopra R, Martel AL (2013) Calculation of intravascular signal in dynamic contrast enhanced-MRI using adaptive complex independent component analysis. IEEE Trans Med Imaging 32:699–710. doi:10.1109/TMI.2012.2233747
Koh TS, Thng CH, Ho JT, Tan PH, Rumpel H, Khoo JB (2008) Independent component analysis of dynamic contrast-enhanced magnetic resonance images of breast carcinoma: a feasibility study. J Magn Reson Imaging 28:271–277. doi:10.1002/jmri.21391
Preul MC, Leblanc R, Caramanos Z, Kasrai R, Narayanan S, Arnold DL (1998) Magnetic resonance spectroscopy guided brain tumor resection: differentiation between recurrent glioma and radiation change in two diagnostically difficult cases. Can J Neurol Sci 25:13–22
Chernov MF, Hayashi M, Izawa M, Usukura M, Yoshida S, Ono Y, Muragaki Y, Kubo O, Hori T, Takakura K (2006) Multivoxel proton MRS for differentiation of radiation-induced necrosis and tumor recurrence after gamma knife radiosurgery for brain metastases. Brain Tumor Pathol 23:19–27. doi:10.1007/s10014-006-0194-9
Weber MA, Giesel FL, Stieltjes B (2008) MRI for identification of progression in brain tumors: from morphology to function. Expert Rev Neurother 8:1507–1525. doi:10.1586/14737175.8.10.1507
Sundgren PC (2009) MR spectroscopy in radiation injury. AJNR Am J Neuroradiol 30:1469–1476. doi:10.3174/ajnr.A1580
Delrue LJ, Casneuf V, Van Damme N, Blanckaert P, Peeters M, Ceelen WP, Duyck PC (2011) Assessment of neovascular permeability in a pancreatic tumor model using dynamic contrast-enhanced (DCE) MRI with contrast agents of different molecular weights. Magma 24:225–232. doi:10.1007/s10334-011-0256-9
Acknowledgments
To Vicki Myers for editorial assistance and Faina Vitinshtein for assistance in patient recruitment and MRI scans.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Artzi, M., Liberman, G., Nadav, G. et al. Differentiation between treatment-related changes and progressive disease in patients with high grade brain tumors using support vector machine classification based on DCE MRI. J Neurooncol 127, 515–524 (2016). https://doi.org/10.1007/s11060-016-2055-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2055-7