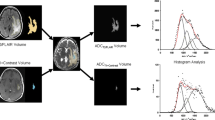
Abstract
Objectives
Treatment-induced lesions represent a great challenge in neuro-oncology. The aims of this study were (i) to characterize treatment induced lesions in glioblastoma patients treated with chemoradiotherapy and heat-shock protein (HSP) vaccine and (ii) to evaluate the diagnostic accuracy of diffusion weighted imaging for differentiation between treatment-induced lesions and tumor progression.
Methods
Twenty-seven patients with newly diagnosed glioblastoma treated with HSP vaccine and chemoradiotherapy were included. Serial magnetic resonance imaging evaluation was performed to detect treatment-induced lesions and assess their growth. Quantitative analysis of the apparent diffusion coefficient (ADC) was performed to discriminate treatment-induced lesions from tumor progression. Mann–Whitney U-test and receiver operating characteristic (ROC) curves were used for analysis.
Results
Thirty-three percent of patients developed treatment-induced lesions. Five treatment-related lesions appeared between end of radiotherapy and the first vaccine administration; 4 lesions within the first 4 months from vaccine initiation and 1 at 3.5 years. Three patients with pathology proven treatment-induced lesions showed a biphasic growth pattern progressed shortly after. ADC ratio between the peripheral enhancing rim and central necrosis showed an accuracy of 0.84 (95% CI 0.63–1) for differentiation between progression and treatment-induced lesions.
Conclusion
Our findings do not support the iRANO recommendation of a 6-month time window in which progressive disease should not be declared after immunotherapy initiation. A biphasic growth pattern of pathologically proven treatment-induced lesions was associated with a dismal prognosis. The presence of lower ADC values in the central necrotic portion of the lesions compared to the enhancing rim shows high specificity for detection of treatment-induced lesions.
Similar content being viewed by others
Introduction
An autologous polyvalent vaccine was evaluated in glioblastoma patients using heat-shock protein (HSP)-peptide complexes derived from autologous glioblastoma tissue [1, 2]. HSP vaccines stimulate antigen uptake by antigen-presenting cells leading to T-lymphocyte activation [3]. This induces both adaptive and innate immune responses against the tumor [4]. Adjuvant treatment of glioblastoma with HSP-peptide vaccine has shown promising results in phase I and II clinical trials [1, 2, 5].
Inflammatory lesions mimicking tumor progression are common after chemoradiotherapy in patients with glioblastoma (often termed “pseudoprogression”). The administration of vaccines and other immunotherapies may exacerbate this problem, as their goal is the induction of an inflammatory response against the tumor. The accurate differentiation of treatment-induced lesions from tumor progression is essential in order to avoid unnecessary surgeries and discontinuation of potentially effective therapies. The immunotherapy Response assessment in Neuro-oncology (iRANO) guidelines were recently published [6]. Recognizing that their recommendations are somewhat empirical, the iRANO guidelines stipulate that, within the first 6 months of immunotherapy, a 3-month window for confirmation of progression is warranted. To date, there are only a few studies that have evaluated and reported the appearance of treatment-induced lesions in pediatric [7, 8] and adult [9, 10] glioma patients treated with vaccine.
Radiological differentiation between treatment-induced lesions and tumor progression remains a clinical dilemma that has not been completely solved despite many advances in imaging techniques and predictive modelling strategies. A recently published study in patients with suspected recurrent glioma [11] describes the presence of restricted diffusion within the central necrotic region of treatment-related necrosis in patients treated with standard of care therapies. However, it is unknown whether immune infiltration after therapeutic vaccine administration can influence the ADC pattern [12].
The aims of this study were, first, to define the incidence, chronology and kinetics of treatment-induced lesions in glioblastoma patients treated with chemoradiotherapy and HSP-vaccine in order to assess the iRANO criteria in this cohort of patients and, second, to evaluate the diagnostic accuracy of diffusion weighted imaging (DWI) for differentiation between treatment-induced lesions and tumor progression.
Materials and methods
This retrospective study was approved by the institutional review board.
Study design
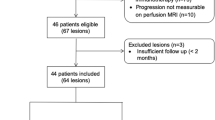
This study includes an institutional cohort of 27 newly diagnosed glioblastoma patients treated with autologous, tumor-derived, HSP-peptide complex and adjuvant temozolomide. The patients were part of a phase II, multi-center, single-arm trial including 46 patients [2] (ClinicalTrials.gov No. NCT00905060). All patients underwent maximal safe resection with intra-operative collection of tissue to generate autologous vaccine. Relevant inclusion criteria for the HSP-vaccine trial were newly diagnosed glioblastoma patients with gross total resection of the contrast-enhancing lesion, sufficient tumor tissue collected to generate a minimum of 4 doses of vaccine, post-operative Karnofsky Performance Scale ≥ 70 and lack of progression after completion of radiotherapy. The complete list of inclusion criteria can be found in a prior publication [2]. Vaccine administration started 2 to 5 weeks after completion of radiotherapy and continued until the vaccine was depleted or progression occurred. Magnetic resonance imaging (MRI) and clinical evaluations were performed approximately every 8 weeks to monitor response and were continued for the full study period (up to 24 months from surgery) or until disease progression. After this, patients were followed with MRI studies timed at the discretion of the patient’s neuro-oncologist.
MRI acquisition
All scans were obtained using a 3.0 T MR scanner (GE Healthcare, Milwaukee, WI), using the body coil for transmission and an 8-channel phased array coil for reception. Images that were evaluated included: DWI with 6-directional axial diffusion EPI sequences (TR/TE = 7000–12,425/76–89 ms, matrix = 256 × 256 × 120, slice thickness = 1.5 mm, FOV = 24 cm × 24 cm × 18 cm, b = 1000 s/mm2, NEX = 4) or DWI with 3-directional axial EPI sequences (TR/TE = 13,800/80.2 ms, matrix = 110 × 116, slice thickness = 2.5 mm, FOV = 25 cm × 22.5, b = 1000 s/mm2, NEX = 4). Volumetric T1-weighted inversion recovery spoiled gradient echo images (TR/TE = 8.86/2.50 ms, matrix = 256 × 256, slice thickness = 1.5 mm, FOV = 24 × 24 cm, TI = 400 ms, Flip angle = 15°) before and after a 5 ml/s bolus injection of 0.1 mmol/kg body weight Gd-DTPA (Magnevist, gadopentetate dimeglumine). T2*-weighted EPI (TE/flip angle = 25–45 ms/35°, matrix, slice thickness = 5 mm).
Imaging interpretation
The presence of treatment-induced lesions was confirmed either by histopathological examination or serial follow-up imaging evaluation by consensus of 2 neuroradiologists with 4 and 9 years of experience in neuroradiology (JEV-M and PA-L). Treatment-induced lesions were defined as new or worsening enhancing lesions according to iRANO criteria showing complete resolution in subsequent scans or histopathologic examination confirming treatment effect with amount of viable tumor ≤ 25% of the tissue [13]. New or worsening enhancing lesions showing progressive imaging findings on subsequent scans or > 25% of viable tumor on histopathology were classified as tumor progression.
Assessment of tumor kinetics
A neuroradiologist (PA-L) manually segmented the enhancing lesion component of treatment-induced lesions appearing within the first 6 months after radiotherapy. The enhancing lesions were contoured on every slice of the T1 post-contrast excluding necrosis and non-enhancing lesion components. We retrospectively applied the iRANO criteria using a ≥ 40% volumetric change as threshold for progressive disease as suggested by the modified RANO criteria for glioblastoma clinical trials [14]. We interpreted each scan together with the clinical notes and we took into account the presence of significant clinical worsening when applying the iRANO radiological criteria. We determined the number of patients meeting the criteria for preliminary progressive disease and quantified the growth of the lesions in the subsequent 3 months. If an MRI was not performed at the 3-month timepoint, the corresponding volume of contrast enhancing lesion was estimated using the scans immediately before and after the 3-month timepoint.
Diffusion imaging analysis
Quantitative ADC evaluation was performed on the subset of lesions showing central necrosis. The first scan showing an area of central necrosis was selected for analysis. Lesions with excessive susceptibility artifact on the T2*-weighted gradient-echo sequence or lack of quantifiable ADC map were excluded. ADC maps were aligned to the post contrast T1 images through rigid body transformations [15]. ROIs encompassing the peripheral enhancing ring, the central necrotic region and the normal appearing white matter were drawn on post contrast T1 images. ROIs were then transferred to the aligned ADC maps for extraction of summary metrics. The open source software package 3D Slicer 4.8 [16] was used for image analysis. Normalized ADC of the central necrotic region and peripheral enhancing lesion were calculated by dividing the mean ADC value of each region by that of the normal appearing white matter. We also calculated the ADC ratio between the enhancing periphery and central necrotic region. The distributions between the different ADC metrics were compared between treatment-induced lesions and tumor progression by using the Mann–Whitney U test. The accuracy of the peripheral/central ADC ratio for detecting treatment-related lesions was estimated by using a receiver operating characteristic (ROC) curve.
Results
Twenty-seven patients were included in the study (12 females and 15 males) with a mean age at diagnosis of 55 (range = 30–66). Twenty-six patients developed a new or worsening enhancing lesion, 9 of which corresponded to treatment-induced lesions and 15 to tumor progression. The outcome of 2 lesions was inconclusive due to bevacizumab administration or lack of follow-up scans. One patient remained stable without evidence of progression as of 7.2 years from surgery. In 5 patients, treatment-induced lesions originated before vaccine was administered; in 3 patients, within the first 4 months after first vaccine administration (4.8 months from end of radiotherapy); and in 1 patient, at 3.5 years. Six treatment-induced lesions and 5 lesions corresponding to tumor progression were confirmed by histopathological examination, the rest were confirmed by imaging follow-up. Only 1 patient presented significant clinical worsening within the first 6 months after immunotherapy initiation as well as marked radiological worsening.
Assessment of tumor kinetics
Growth rates of lesions corresponding to early treatment-induced lesions and early tumor progression were variable (Fig. 1). According to iRANO, 7 patients met the criteria for preliminary progressive disease. Three patients met the criteria at 2 months; 1 at 3 months; and 3 slightly before 4 months after first vaccine administration. Results of lesion growth evaluation after 3 months from declaration of preliminary progressive disease were as follows: 3 patients had surgery before the 3-month timepoint and the 4 patients whose lesions were not resected showed either improvement (negative volumetric change of − 28% and − 95%) or growth below the 40% threshold (6% and 22%). In 3 cases we observed a biphasic evolution of treatment-induced lesions with further worsening after a period of stabilization or improvement. These 3 patients underwent reoperation and, despite being classified as treatment-induced lesions by histopathology, all 3 developed confirmed radiologic progression within 2.1 months after the second surgery. These 3 patients also showed shorter survival times than the median overall survival of the cohort (26 months).
Evolution of the contrast enhancing lesion volume over time in cases of early treatment-induced lesions and retrospective application of iRANO criteria. All cases of early treatment-induced lesions start before 4 months post vaccine initiation. In 3 cases of treatment-induced lesions, a biphasic pattern is observed with a second period of worsening after stabilization or improvement (dashed lines). This biphasic patter was associated to bad prognosis despite histopathological diagnosis of treatment-induced lesions
Diffusion imaging analysis
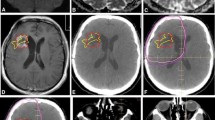
All 9 treatment-induced lesions were included for quantitative analysis. Of the 15 lesions corresponding to tumor progression, 7 lesions were included for quantitative ADC analysis after excluding 5 lesions due to lack of central necrosis, 2 due to susceptibility artifact and 1 due to lack of ADC map (Fig. 2). Values of the different ADC ratios are shown in Table 1. There was a significant difference (p = 0.023) in the peripheral/central ADC ratio between treatment-induced lesions and tumor progression (Fig. 3a). No significant difference was detected in normalized ADC of the peripheral enhancing or central regions between treatment-induced lesions and tumor progression. Area under the ROC curve (Fig. 3b) of the peripheral/central ADC ratio was 0.84 (95% CI 0.63–1). The cutoff value of 1.06 showed maximal accuracy with a sensitivity for detection of treatment-induced lesions of 78% and a specificity of 100%. Examples of ADC patterns in treatment-induced lesions and in progressive disease are shown in Fig. 4.
a Box and whiskers plot depicting the distributions of the peripheral/central ADC ratio in the tumor progression and treatment-induced lesions groups (p = 0.023). b Receiver operating characteristic curve for the detection of treatment-induced lesions by using the peripheral/central ADC ratio (positive state corresponds to treatment-induced lesion)
Examples depicting the inverted ADC pattern in treatment-induced lesions compared to progressive tumor. Lower ADC values are centrally located in the treatment-induced lesion depicted in the upper row versus peripherally in the case of tumor progression in the lower row. Histopathological evaluation in the case featured in the upper row showed necrotic tissue without recurrent neoplasm. The patient was alive 3 years after this scan. In the case featured in the lower row, histopathological evaluation showed mostly viable tumor and the patient died 1 year after this scan
Discussion
Thirty-three percent of the study participants developed treatment-induced lesions. Five patients developed treatment-induced lesions before vaccine administration and 3 patients within the first 4 months from vaccine initiation. Only one patient developed a delayed treatment-induced lesion at 3.5 years. When the iRANO criteria were retrospectively applied to patients with early treatment-induced lesions, 7 patients met the criteria for preliminary progressive disease. Three of these 7 patients underwent reoperation before the 3-month window established to confirm progressive disease. The 4 remaining patients showed improvement or low growth rates at 3 months and they would have been adequately classified as treatment-induced lesions by iRANO criteria. Reduced diffusion within the central necrotic region of a new or worsening enhancing lesion compared to the enhancing component showed high specificity for the diagnosis of treatment-induced lesions.
Most treatment-induced lesions in our cohort occurred within the first 5 months after radiotherapy completion. According to the literature, most cases of treatment-induced lesions in glioblastoma patients treated with chemoradiotherapy are noted within the first 3–4 months after the end of radiotherapy, although delayed cases have also been described [17]. Five cases of treatment-induced lesions occurred before the first vaccine administration, which is expected, as it has been reported that 20–30% of glioblastoma patients show increased contrast enhancement in their first post-radiation MRI with subsequent improvement [18]. Evidence suggests that radiotherapy effects extend beyond the mere elimination of the most radiosensitive fraction of tumor cells. Local radiation enhances the susceptibility of solid tumors to immune-mediated destruction, perhaps by facilitating the penetration and function of dendritic cells and effector T cells [19]. In the present study, the vaccine was administered between 2 and 5 weeks after the end of radiotherapy and may have affected the subsequent evolution of treatment-induced lesions compared with patients treated with chemoradiotherapy alone. Given the possible synergistic effect of chemoradiotherapy and immunotherapy and the proximity in time in which they were administered, it is difficult to disentangle the contribution of these treatments in the occurrence and evolution of treatment-induced lesions. A few studies in glioma patients treated with vaccine investigated the development of treatment-induced lesions. A dendritic cell vaccine trial on recurrent gliomas in adults reported an incidence of 4.5% [10]. Similarly, a study in children with recurrent low-grade gliomas treated with glioma-associated antigen peptide vaccination found an incidence of 7.1% [20], whereas a higher incidence (19%) was reported in a glioma-associated antigen peptide vaccine trial in newly diagnosed pediatric gliomas [8]. We believe the higher incidence of treatment-induced lesions found in vaccination studies for newly diagnosed gliomas compared to studies in recurrent gliomas is likely related to the associated chemoradiotherapy administration.
In order to address the issue of treatment-induced lesions, the iRANO guidelines suggest a 6-month period after the start of immunotherapy in which progressive disease needs to be confirmed by a follow up scan. In our cohort, there were no cases of early treatment-induced lesions occurring after 4 months from vaccine initiation, so our results support a reduction of the 6-month time window. We found 3 cases of pathologically proven treatment-induced lesions with biphasic volumetric evolution showing true tumor progression within 2.1 months of the second surgery. We believe this discordance is attributable to sampling error with failure to capture areas of recurrent tumor on a background of treatment effect. Although all patients underwent open surgery with either subtotal or gross total resection, not all areas of excised tissue are routinely evaluated by the pathologist. In patients with biphasic lesion growth, histopathology showing treatment effect with none or small amount of viable tumor, did not seem to be a reliable outcome predictor. To our knowledge, dynamics of treatment-induced lesions have not been previously investigated. Our findings, although in a small sample of patients, suggest that true treatment-induced lesions have a monophasic course and that a biphasic growth pattern, carries a dismal prognosis despite histopathological diagnosis of treatment effect.
Prior studies evaluating ADC for differentiation between progression and treatment-induced lesions show inconsistent results. In general, the average ADC value of the contrast enhancing region has shown moderate diagnostic accuracy for the differentiation between these two entities [21, 22]. In order to account for the heterogeneity of lesions, some authors resort to histogram analysis of ADC. Some studies found that the smallest ADC values within the enhancing lesion are the most useful [23], other studies used intermediate ADC [24] and another study assigned the best discriminative power to the maximum ADC values [25]. A recent meta-analysis found moderate diagnostic performance of diffusion MRI in differentiating glioma recurrence from radiation necrosis and recommends a multimodal approach. The presence of centrally restricted diffusion in treatment-induced lesions can explain the inconsistent results found in the literature. Most studies focus on the ADC of the enhancing component, not realizing that the central coagulative necrosis in treatment-induced lesions can restrict water motion to the same degree or even higher than tumor cellularity [26]. A recent article by Zakhari et al. [11] describes the presence of centrally restricted diffusion in treatment induced necrosis and the diagnostic accuracy of the central/peripheral ADC ratio for differentiation between radiation necrosis and recurrent tumor. Our findings, with a similar number of patients, support their results and consolidate the finding of centrally reduced diffusion as a highly specific sign of treatment-related lesions.
The current study has three main limitations. First, it included a small number of subjects and needs to be validated in a larger cohort. Second, only one neuroradiologist performed the tumor segmentation. Although this is usually the case in imaging research studies where manual segmentations are required, the lack of interobserver agreement assessment should be acknowledged. Third, the steroid dose was not directly available in the clinical notes. However, neuro-oncologists at our institution take this into account when assessing the results of the MRI and would make it clear in the clinical notes if there is suspicion that the radiological worsening is related to change in steroid dose. Nevertheless, we acknowledge that any information that is not systematically collected is susceptible of being overlooked.
Conclusion
One in three patients with newly diagnosed glioblastoma treated with chemoradiotherapy and HSP vaccine developed treatment-induced lesions. This proportion is not very different to that reported in studies involving standard of care chemoradiotherapy. Our findings do not support the iRANO 6-month window in which progressive disease should not be declared after immunotherapy initiation and further evidence-based refinements to this time period are warranted. We found that a biphasic pattern of growth in pathologically confirmed treatment-related lesions was associated with a dismal prognosis. The presence of lower ADC values in the central necrotic portion of the lesion compared to the enhancing rim is a specific sign of treatment-induced lesions. Further studies assessing tumor kinetics and incorporating advanced imaging in glioma immunotherapy will be essential in order to further refine response assessment guidelines.
References
Bloch O, Parsa AT (2014) Heat shock protein peptide complex-96 (HSPPC-96) vaccination for recurrent glioblastoma: a phase II, single arm trial. Neuro Oncol 16:758–759. https://doi.org/10.1093/neuonc/nou054
Bloch O, Lim M, Sughrue ME et al (2017) Autologous heat shock protein peptide vaccination for newly diagnosed glioblastoma: impact of peripheral PD-L1 expression on response to therapy. Clin Cancer Res 23:3575–3584. https://doi.org/10.1158/1078-0432.CCR-16-1369
Murshid A, Gong J, Stevenson MA, Calderwood SK (2011) Heat shock proteins and cancer vaccines: developments in the past decade and chaperoning in the decade to come. Expert Rev Vaccines 10:1553–1568. https://doi.org/10.1586/erv.11.124
Ampie L, Choy W, Lamano JB et al (2015) Heat shock protein vaccines against glioblastoma: from bench to bedside. J Neurooncol 123:441–448. https://doi.org/10.1007/s11060-015-1837-7
Ji N, Zhang Y, Liu Y et al (2018) Heat shock protein peptide complex-96 vaccination for newly diagnosed glioblastoma: a phase I, single-arm trial. JCI Insight. https://doi.org/10.1172/jci.insight.99145
Okada H, Weller M, Huang R et al (2015) Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol 16:e534–e542. https://doi.org/10.1016/s1470-2045(15)00088-1
Ceschin R, Kurland BF, Abberbock SR et al (2015) Parametric response mapping of apparent diffusion coefficient as an imaging biomarker to distinguish pseudoprogression from true tumor progression in peptide-based vaccine therapy for pediatric diffuse intrinsic pontine glioma. Am J Neuroradiol 36:2170–2176. https://doi.org/10.3174/ajnr.A4428
Pollack IF, Jakacki RI, Butterfield LH et al (2014) Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and n. J Clin Oncol 32:2050–2058. https://doi.org/10.1200/JCO.2013.54.0526
Sampson JH, Heimberger AB, Archer GE et al (2010) Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol 28:4722–4729. https://doi.org/10.1200/JCO.2010.28.6963
Okada H, Kalinski P, Ueda R et al (2011) Induction of CD8 + T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in. J Clin Oncol 29:330–336. https://doi.org/10.1200/jco.2010.30.7744
Zakhari N, Taccone MS, Torres C et al (2018) Diagnostic accuracy of centrally restricted diffusion in the differentiation of treatment-related necrosis from tumor recurrence in high-grade gliomas. Am J Neuroradiol 39:260–264
Sokratous G, Polyzoidis S, Ashkan K (2017) Immune infiltration of tumor microenvironment following immunotherapy for glioblastoma multiforme. Hum Vaccines Immunother 13:2575–2582. https://doi.org/10.1080/21645515.2017.1303582
Wang S, Sakai Y, Chawla S et al (2016) Differentiating tumor progression from pseudoprogression in patients with glioblastomas using diffusion tensor imaging and dynamic susceptibility contrast MRI. Am J Neuroradiol. https://doi.org/10.3174/ajnr.A4474
Ellingson BM, Wen PY, Cloughesy TF (2017) Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics 14:307–320. https://doi.org/10.1007/s13311-016-0507-6
Maes F, Collignon A, Vandermeulen D et al (1997) Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging 16:187–198. https://doi.org/10.1109/42.563664
Fedorov A, Beichel R, Kalpathy-Cramer J et al (2012) 3D slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging 30:1323–1341. https://doi.org/10.1016/j.mri.2012.05.001
Nasseri M, Gahramanov S, Netto JP et al (2014) Evaluation of pseudoprogression in patients with glioblastoma multiforme using dynamic magnetic resonance imaging with ferumoxytol calls RANO criteria into question. Neuro Oncol 16:1146–1154. https://doi.org/10.1093/neuonc/not328
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. https://doi.org/10.1200/JCO.2009.26.3541
Demaria S, Bhardwaj N, McBride WH, Formenti SC (2005) Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol Biol Phys 63:655–666. https://doi.org/10.1016/j.ijrobp.2005.06.032
Pollack IF, Jakacki RI, Butterfield LH et al (2016) Immune responses and outcome after vaccination with glioma-associated antigen peptides and poly-ICLC in a pilot study for pediatric recurrent low-grade gliomas†. Neuro Oncol 18:1157–1168. https://doi.org/10.1093/neuonc/now026
Bobek-Billewicz B, Stasik-Pres G, Majchrzak H, Zarudzki L (2010) Differentiation between brain tumor recurrence and radiation injury using perfusion, diffusion-weighted imaging and MR spectroscopy. Folia Neuropathol 48:81–92
Lee WJ, Choi SH, Park CK et al (2012) Diffusion-weighted MR imaging for the differentiation of true progression from pseudoprogression following concomitant radiotherapy with temozolomide in patients with newly diagnosed high-grade gliomas. Acad Radiol 19:1353–1361. https://doi.org/10.1016/j.acra.2012.06.011
Matsusue E, Fink JR, Rockhill JK et al (2010) Distinction between glioma progression and post-radiation change by combined physiologic MR imaging. Neuroradiology 52:297–306. https://doi.org/10.1007/s00234-009-0613-9
Qin L, Li X, Stroiney A et al (2017) Advanced MRI assessment to predict benefit of anti-programmed cell death 1 protein immunotherapy response in patients with recurrent glioblastoma. Neuroradiology 59:135–145. https://doi.org/10.1007/s00234-016-1769-8
Asao C, Korogi Y, Kitajima M et al (2005) Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. Am J Neuroradiol 26:1455–1460
Wang S, Chen Y, Lal B et al (2012) Evaluation of radiation necrosis and malignant glioma in rat models using diffusion tensor MR imaging. J Neurooncol 107:51–60. https://doi.org/10.1007/s11060-011-0719-x
Acknowledgements
This work was supported by the National Institutes of Health (P01 CA118816). We would like to thank Sarah Nelson for her support.
Funding
This study was funded by the National Institutes of Health (P01 CA118816).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Paula Alcaide Leon, Marisa Lafontaine, Janine M. Lupo, Hideho Okada, Jennifer L. Clark and Javier E. Villanueva-Meyer declares that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alcaide-Leon, P., Luks, T.L., Lafontaine, M. et al. Treatment-induced lesions in newly diagnosed glioblastoma patients undergoing chemoradiotherapy and heat-shock protein vaccine therapy. J Neurooncol 146, 71–78 (2020). https://doi.org/10.1007/s11060-019-03336-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03336-3