Abstract
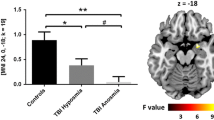
Upper respiratory tract infection (URI) is one of the most common etiology of olfactory loss. Previous studies demonstrated that both olfactory bulb (OB) volume and sulcus (OS) depth decreased in patients with post-infectious olfactory loss (PIOL) compared to normal controls. The aim of our study was to observe alterations of central olfactory pathways in patients with PIOL. T1 weighted magnetic resonance images were acquired in 19 PIOL patients and 19 age- and sex-matched control subjects on a 3 T scanner. Voxel-based morphometry (VBM) was performed using VBM8 toolbox and SPM8 in a Matlab environment. We also analyzed OB volume in coronal T2-weighted images. Whole-brain analysis revealed a significant gray matter volume loss in the right orbitofrontal cortex (OFC) in patients group. Further analysis with region of interest exhibited a significant negative correlation between gray matter volume in right OFC as well as OB volume and the duration of olfactory loss in these patients (r = -0.566 and r = -0.535 both P < 0.05, respectively). In conclusion, the morphological alterations in the right OFC and OB might contribute to the pathogenic mechanism of olfactory dysfunction after upper respiratory tract infection.


Similar content being viewed by others
References
Ashburner, J., & Friston, K. J. (2000). Voxel-based morphometry-the methods. Neuroimage, 11(6 Pt1), 805–821.
Bitter, T., Brüderle, J., Gudziol, H., Burmeister, H. P., Gaser, C., & Guntinas-Lichius, O. (2010b). Gray and white matter reduction in hyposmic subjects–A voxelbased morphometry study. Brain Research, 1347, 42–47.
Bitter, T., Gudziol, H., Burmeister, H. P., Mentzel, H. J., & Guntinas-Lichius, O. Gaser C. (2010a). Anosmia leads to a loss of gray matter in cortical brain areas. Chemical Senses. 35(5):407–415.
Bitter, T., Siegert, F., Gudziol, H., Burmeister, H. P., Mentzel, H. J., Hummel, T., Gaser, C., & Guntinas-Lichius, O. (2011). Gray matter alterations in parosmia. Neuroscience, 177, 177–182.
Bookstein, F. L. (2001). “Voxel-based morphometry” should not be used with imperfectly registered images. Neuroimage, 14(6), 1454–1462.
Boyett-Anderson, J. M., Lyons, D. M., Reiss, A. L., Schatzberg, A. F., & Menon, V. (2003). Functional brain imaging of olfactory processing in monkeys. Neuroimage. 20(1):257 – 64.
Chen, G., Wei, Y., Miao, X., Li, K., Ren, Y., & Liu, J. (2013). Clinical features of olfactory disorders in patients seeking medical consultation. Medical Science Monitor, 19, 444–450.
Cockrell, J. R., & Folstein, M. F. (1988). Mini-Mental State Examination (MMSE). Psychopharmacology Bulletin. 24(4):689 – 92.
Delon-Martin, C., Plailly, J., Fonlupt, P., Veyrac, A., & Royel, J. P. (2013). Perfumers’ expertise induces structural reorganization in olfactory brain regions. Neuroimage, 68, 55–62.
Frasnelli, J., Fark, T., Lehmann, J., Gerber, J., & Hummel, T. (2013). Brain structure is changed in congenital anosmia. Neuroimage, 83, 1074–1080.
Frasnelli, J., Lundström, J. N., Boyle, J. A., Djordjevic, J., & Zatorre, R. J. Jones-Gotman M. (2010). Neuroanatomical correlates of olfactory performance. Experimental Brain Research. 201(1):1–11.
Gottfried, J. A., & Zald, D. H. (2005). On the scent of human olfactory orbitofrontal cortex: meta-analysis and comparison to non-human primates. Brain Research. Brain Research Reviews, 50, 287–304.
Gottfried, J. A., & Zelano, C. (2011). The value of identity: olfactory notes on orbitofrontal cortex function. Annals of the New York Academy of Sciences. 1239: 138 – 48.
Gudziol, V., Paech, I., & Hummel, T. (2010). Unilateral reduced sense of smell is an early indicator for global olfactory loss. Journal of Neurology, 257(6), 959 – 63.
Hedner, M., Larsson, M., Arnold, N., Zucco, G. M., & Hummel, T. (2010). Cognitive factors in odor detection, odor discrimination, and odor identification tasks. Journal of Clinical and Experimental Neuropsychology, 32(10), 1062–1067.
Hummel, T., Sekinger, B., Wolf, S. R., Pauli, E., & Kobal, G. (1997). ‘Sniffin’ sticks’: Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chemical Senses, 22, 39–52.
Hummel, T., Urbig, A., Huart, C., Duprez, T., & Rombaux, P. (2015). Volume of olfactory bulb and depth of olfactory sulcus in 378 consecutive patients with olfactory loss. Journal of Neurology, 262(4), 1046–1051.
Jafek, B. W., Murrow, B., Michaels, R., Restrepo, D., & Linschoten, M. (2002). Biopsies of human olfactory epithelium. Chemical Senses, 27(7), 623–628.
Kobal, G., Hummel, T., Sekinger, B., Barz, S., Roscher, S., & Wolf, S. (1996). “Sniffin’ sticks”: Screening of olfactory performance. Rhinology, 34, 222–226.
Oldfield, R. C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9, 97–113.
Patterson, A., Hähner, A., Kitzler, H. H., & Hummel, T. (2015). Are small olfactory bulbs a risk for olfactory loss following an upper respiratory tract infection? European Archives of Oto-Rhino-Laryngology, 272(11), 3593–3594.
Potter, H., & Butters, N. (1980). An assessment of kolfactory deficits in patients with damage to prefrontal cortex. Neuropsychologia, 18, 621–628.
Rombaux, P., Mouraux, A., Bertrand, B., Nicolas, G., Duprez, T., & Hummel, T. (2006). Olfactory function and olfactory bulb volume in patients with postinfectious olfactory loss. Laryngoscope, 116(3), 436–439.
Seiden, A. M. (2004). Postviral olfactory loss. Otolaryngologic Clinics of North America, 37(6), 1159–1166.
Seiden, A. M., & Duncan, H. J. (2001). The diagnosis of a conductive olfactory loss. Laryngoscope, 111(1), 9–14.
Seubert, J., Freiherr, J., Frasnelli, J., Hummel, T., & Lundström, J. N. (2013). Orbitofrontal cortex and olfactory bulb volume predict distinct aspects of olfactory performance in healthy subjects. Cerebral Cortex, 23(10), 2448–2456.
Temmel, A. F., Quint, C., Schickinger-Fischer, B., Klimek, L., Stoller, E., & Hummel, T. (2002). Characteristics of olfactory disorders in relation to major causes of olfactory loss. Archives of Otolaryngology - Head and Neck Surgery, 128(6), 635 – 41.
Welge-Lüssen, A., Gudziol, V., Wolfensberger, M., & Hummel, T. (2010). Olfactory testing in clinical setting - is there additional benefit from unilateral testing? Rhinology. 48(2):156–159.
Willhite, D. C., Nguyen, K. T., Masurkar, A. V., Greer, C. A., Shepherd, G. M., & Chen, W. R. (2006). Viral tracing identifies distributed columnar organization in the olfactory bulb. Proceedings of the National Academy of Sciences of the United States of America, 103(33), 12592–12597.
Yamagishi, M., Fujiwara, M., Nakamura, H. (1994). Olfactory mucosal findings and clinical course in patients with olfactory disorders following upper respiratory viral infection. Rhinology, 32(3), 113–118.
Yang, L., Wei, Y., Yu, D., Zhang, J., & Liu, Y. (2010). Olfactory and gustatory function in healthy adult Chinese subjects. Otolaryngol Head Neck Surg. 143: 554 – 60.
Yao, L., Pinto, J. M., Yi, X., Li, L., Peng, P., & Wei, Y. (2014). Gray matter volume reduction of olfactory cortices in patients with idiopathic olfactory loss.Chem Senses. 39(9):755 – 60.
Yao, L., Yi, X., & Wei, Y. (2013). Gray matter alteration in isolated congenital anosmia patient: a voxel-based morphometry study. European Archives of Oto-Rhino-Laryngology, 270(9), 2569–2573.
Yousem, D. M., Maldjian, J. A., Siddiqi, F., Hummel, T., Alsop, D. C., Geckle, R. J., Bilker, W. B., & Doty, R. L. (1999). Gender effects on odor-stimulated functional magnetic resonance imaging. Brain Research, 818(2), 480–487.
Zald, D. H., & Pardo, J. V. (2000). Functional neuroimaging of the olfactory system in humans. International Journal of Psychophysiology, 36(2), 165 – 81.
Zatorre, R. J., Jones-Gotman, M., Evans, A. C., & Meyer, E. (1992). Functional localization and lateralization of human olfactory cortex. Nature. 360(6402):339 – 40.
Acknowledgements
We would like to thank Thomas Hummel for valuable assistance on the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China [81,670,903] and the Foundation of Beijing Anzhen Hospital, Capital Medical University (No.2016Z02).
Author information
Authors and Affiliations
Contributions
L. Yao and X. Yi, substantial contributions to conception and design, drafting the article and revising it critically for important intellectual content, acquisition of data, analysis and interpretation of data, and final approval of the version to be published; Y. Wei, substantial contributions to conception and design, analysis and interpretation of data, and final approval of the version to be published; J.M. Pinto, analysis and interpretation of data, revising the article, and final approval of the version to be published; Y. Guo and Y. Liu, who were the observers to measure OB volume, contribution to acquisition of data and final approval of the version to be published; X. Yuan, who was the third referee to measure OB volume, contributions to acquisition of data and final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no financial interests or potential conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Yao, L., Yi, X., Pinto, J.M. et al. Olfactory cortex and Olfactory bulb volume alterations in patients with post-infectious Olfactory loss. Brain Imaging and Behavior 12, 1355–1362 (2018). https://doi.org/10.1007/s11682-017-9807-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-017-9807-7