Abstract
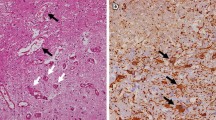
Radiation injury to the central nervous system (CNS) manifests in multiple forms and is divided into three categories termed acute, early-delayed, and late-delayed injury patterns. Late-delayed radiation injury, primarily manifesting as leukoencephalopathy or radiation necrosis, is often progressive and may have a negative impact on quality of life. Radiation injury is believed to be a consequence of cell membrane and DNA injury with a pathological expression as vascular injury, depletion of oligodendroglial progenitor cells, and failure of cell-cell interactions that constitute the cellular network of the CNS. In addition, radiation injury results in activation of the inflammatory cascade with perturbation of cytokines, production of reactive oxygen species and vascular endothelial growth factor. Medical treatment of CNS radiation injury and in particular radiation necrosis remains problematic as there is a paucity of clinical trial data to inform treatment decisions, and aside from surgery and corticosteroids only bevacizumab appears to have a compelling therapeutic role.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Leibel S, Sheline G. Tolerance of the brain and spinal cord to conventional irradiation. In: Gutin PLS, Sheline G, editors. Radiaiton injury to the nervous system. New York: Raven; 1991. p. 239–56.
Young DF, Posner JB, Chu F, Nisce L. Rapid-course radiation therapy of cerebral metastases: results and complications. Cancer. 1974;34(4):1069–76.
Hindo WA, DeTrana 3rd FA, Lee MS, Hendrickson FR. Large dose increment irradiation in treatment of cerebral metastases. Cancer. 1970;26(1):138–41.
Rider WD. Radiation damage to the brain–a new syndrome. J Can Assoc Radiol. 1963;14:67–9.
Hoffman WF, Levin VA, Wilson CB. Evaluation of malignant glioma patients during the postirradiation period. J Neurosurg. 1979;50(5):624–8. doi:10.3171/jns.1979.50.5.0624.
Wilson CB, Crafts D, Levin V. Brain tumors: criteria of response and definition of recurrence. Natl Cancer Inst Monogr. 1977;46:197–203.
Taal W, Brandsma D, de Bruin HG, Bromberg JE, Swaak-Kragten AT, Smitt PA, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113(2):405–10. doi:10.1002/cncr.23562.
de Wit MC, de Bruin HG, Eijkenboom W, Sillevis Smitt PA, van den Bent MJ. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63(3):535–7.
• Brandes AA, Tosoni A, Franceschi E, Sotti G, Frezza G, Amista P, et al. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: correlation with MGMT promoter methylation status. J Clin Oncol. 2009;27(8):1275–9. doi:10.1200/JCO.2008.19.4969. Characterization of pseudoprogression following temozolomide-based chemoradiotherapy.
Kumar AJ, Leeds NE, Fuller GN, Van Tassel P, Maor MH, Sawaya RE, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000;217(2):377–84.
• Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–61. doi:10.1016/S1470-2045(08)70125-6. A hypothesis-generating paper regarding mechanisms of pseudoprogression.
Martins AN, Johnston JS, Henry JM, Stoffel TJ, Di Chiro G. Delayed radiation necrosis of the brain. J Neurosurg. 1977;47(3):336–45. doi:10.3171/jns.1977.47.3.0336.
Yoshii Y, Phillips TL. Late vascular effects of whole brain X-irradiation in the mouse. Acta Neurochir (Wien). 1982;64(1–2):87–102.
Calvo W, Hopewell JW, Reinhold HS, Yeung TK. Time- and dose-related changes in the white matter of the rat brain after single doses of X rays. Br J Radiol. 1988;61(731):1043–52.
Reinhold HS, Calvo W, Hopewell JW, van der Berg AP. Development of blood vessel-related radiation damage in the fimbria of the central nervous system. Int J Radiat Oncol Biol Phys. 1990;18(1):37–42.
Manz HJ, Woolley 3rd PV, Ornitz RD. Delayed radiation necrosis of brainstem related to fast neutron beam irradiation: case report and literature review. Cancer. 1979;44(2):473–9.
Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303(5916):390–6.
Schultheiss TE, Stephens LC. Invited review: permanent radiation myelopathy. Br J Radiol. 1992;65(777):737–53.
Marks JE, Baglan RJ, Prassad SC, Blank WF. Cerebral radionecrosis: incidence and risk in relation to dose, time, fractionation and volume. Int J Radiat Oncol Biol Phys. 1981;7(2):243–52.
Leibel SA, Sheline GE. Radiation therapy for neoplasms of the brain. J Neurosurg. 1987;66(1):1–22.
Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6(9):1215–28.
Ellis F. Dose, time and fractionation: a clinical hypothesis. Clin Radiol. 1969;20(1):1–7.
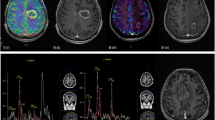
Valk PE, Dillon WP. Radiation injury of the brain. AJNR Am J Neuroradiol. 1991;12(1):45–62.
•• Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–72. doi:10.1200/JCO.2009.26.3541. New and revised brain tumor radiographic response criteria.
• Fink J, Born D, Chamberlain MC. Pseudoprogression: relevance with respect to treatment of high-grade gliomas. Curr Treat Options Oncol. 2011;12(3):240–52. doi:10.1007/s11864-011-0157-1. A contemporary review of pseudoprogression.
Young RJ, Gupta A, Shah AD, Graber JJ, Zhang Z, Shi W, et al. Potential utility of conventional MRI signs in diagnosing pseudoprogression in glioblastoma. Neurology. 2011;76(22):1918–24. doi:10.1212/WNL.0b013e31821d74e7.
Burger PC, Boyko OB. The pathology of central nervous system radiation injury. In: Gutin P, Leibel S, Sheline G, editors. Radiation injury to the nervous system. New York: Raven; 1991. p. 191–208.
Giglio P, Gilbert MR. Cerebral radiation necrosis. Neurologist. 2003;9(4):180–8. doi:10.1097/01.nrl.0000080951.78533.c4.
Perry A, Schmidt RE. Cancer therapy-associated CNS neuropathology: an update and review of the literature. Acta Neuropathol. 2006;111(3):197–212. doi:10.1007/s00401-005-0023-y.
Safdari H, Boluix B, Gros C. Multifocal brain radionecrosis masquerading as tumor dissemination. Surg Neurol. 1984;21(1):35–41.
Schiffer D, Giordana MT, Soffietti R, Sciolla R, Sannazzari GL, Vasario E. Effects of radiotherapy on the astrocytomatous areas of malignant gliomas. J Neurooncol. 1984;2(3):167–75.
Chamberlain MC, Glantz MJ, Chalmers L, Van Horn A, Sloan AE. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol. 2007;82(1):81–3. doi:10.1007/s11060-006-9241-y.
Yaman E, Buyukberber S, Benekli M, Oner Y, Coskun U, Akmansu M, et al. Radiation induced early necrosis in patients with malignant gliomas receiving temozolomide. Clin Neurol Neurosurg. 2010;112(8):662–7. doi:10.1016/j.clineuro.2010.05.003.
Morris GM, Coderre JA, Bywaters A, Whitehouse E, Hopewell JW. Boron neutron capture irradiation of the rat spinal cord: histopathological evidence of a vascular-mediated pathogenesis. Radiat Res. 1996;146(3):313–20.
Mastaglia FL, McDonald WI, Watson JV, Yogendran K. Effects of x-radiation on the spinal cord: an experimental study of the morphological changes in central nerve fibres. Brain. 1976;99(1):101–22.
van der Maazen RW, Kleiboer BJ, Verhagen I, van der Kogel AJ. Irradiation in vitro discriminates between different O-2A progenitor cell subpopulations in the perinatal central nervous system of rats. Radiat Res. 1991;128(1):64–72.
van der Maazen RW, Kleiboer BJ, Verhagen I, van der Kogel AJ. Repair capacity of adult rat glial progenitor cells determined by an in vitro clonogenic assay after in vitro or in vivo fractionated irradiation. Int J Radiat Biol. 1993;63(5):661–6.
van der Maazen RW, Verhagen I, Kleiboer BJ, van der Kogel AJ. Radiosensitivity of glial progenitor cells of the perinatal and adult rat optic nerve studied by an in vitro clonogenic assay. Radiother Oncol. 1991;20(4):258–64.
Hornsey S, Myers R, Coultas PG, Rogers MA, White A. Turnover of proliferative cells in the spinal cord after X irradiation and its relation to time-dependent repair of radiation damage. Br J Radiol. 1981;54(648):1081–5.
Hansson E. Astroglia from defined brain regions as studied with primary cultures. Prog Neurobiol. 1988;30(5):369–97.
Muller HW, Junghans U, Kappler J. Astroglial neurotrophic and neurite-promoting factors. Pharmacol Ther. 1995;65(1):1–18.
Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417(6884):39–44. doi:10.1038/417039a 417039a.
Hong-Brown LQ, Brown CR. Cytokine and insulin regulation of alpha 2 macroglobulin, angiotensinogen, and hsp 70 in primary cultured astrocytes. Glia. 1994;12(3):211–8. doi:10.1002/glia.440120306.
Ijichi A, Sakuma S, Tofilon PJ. Hypoxia-induced vascular endothelial growth factor expression in normal rat astrocyte cultures. Glia. 1995;14(2):87–93. doi:10.1002/glia.440140203.
Iwata-Ichikawa E, Kondo Y, Miyazaki I, Asanuma M, Ogawa N. Glial cells protect neurons against oxidative stress via transcriptional up-regulation of the glutathione synthesis. J Neurochem. 1999;72(6):2334–44.
Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325(6101):253–7. doi:10.1038/325253a0.
Schroeter ML, Mertsch K, Giese H, Muller S, Sporbert A, Hickel B, et al. Astrocytes enhance radical defence in capillary endothelial cells constituting the blood-brain barrier. FEBS Lett. 1999;449(2–3):241–4.
Wilson JX. Antioxidant defense of the brain: a role for astrocytes. Can J Physiol Pharmacol. 1997;75(10–11):1149–63.
Chiang CS, McBride WH, Withers HR. Radiation-induced astrocytic and microglial responses in mouse brain. Radiother Oncol. 1993;29(1):60–8.
Nordal RA, Nagy A, Pintilie M, Wong CS. Hypoxia and hypoxia-inducible factor-1 target genes in central nervous system radiation injury: a role for vascular endothelial growth factor. Clin Cancer Res. 2004;10(10):3342–53. doi:10.1158/1078-0432.CCR-03-0426.
Thomas WE. Brain macrophages: evaluation of microglia and their functions. Brain Res Brain Res Rev. 1992;17(1):61–74.
Vaughan DW, Peters A. Neuroglial cells in the cerebral cortex of rats from young adulthood to old age: an electron microscope study. J Neurocytol. 1974;3(4):405–29.
Giulian D, Chen J, Ingeman JE, George JK, Noponen M. The role of mononuclear phagocytes in wound healing after traumatic injury to adult mammalian brain. J Neurosci. 1989;9(12):4416–29.
Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58(3):233–47.
Mildenberger M, Beach TG, McGeer EG, Ludgate CM. An animal model of prophylactic cranial irradiation: histologic effects at acute, early and delayed stages. Int J Radiat Oncol Biol Phys. 1990;18(5):1051–60.
Nakagawa M, Bellinzona M, Seilhan TM, Gobbel GT, Lamborn KR, Fike JR. Microglial responses after focal radiation-induced injury are affected by alpha-difluoromethylornithine. Int J Radiat Oncol Biol Phys. 1996;36(1):113–23.
Schultheiss TE, Stephens LC, Maor MH. Analysis of the histopathology of radiation myelopathy. Int J Radiat Oncol Biol Phys. 1988;14(1):27–32.
Tofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiat Res. 2000;153(4):357–70.
Kim JH, Brown SL, Jenrow KA, Ryu S. Mechanisms of radiation-induced brain toxicity and implications for future clinical trials. J Neurooncol. 2008;87(3):279–86. doi:10.1007/s11060-008-9520-x.
Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem. 2000;267(16):4912–6.
Smith KJ, Kapoor R, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 1999;9(1):69–92.
Applegate LA, Luscher P, Tyrrell RM. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991;51(3):974–8.
Gutin PH, Levin KJ, McDermott MW, Hooper N, Smith MT, Cashman JR, et al. Lipid peroxidation does not appear to be a factor in late radiation injury of the cervical spinal cord of rats. Int J Radiat Oncol Biol Phys. 1993;25(1):67–72.
Belka C, Budach W, Kortmann RD, Bamberg M. Radiation induced CNS toxicity–molecular and cellular mechanisms. Br J Cancer. 2001;85(9):1233–9. doi:10.1054/bjoc.2001.2100.
• Yoshii Y. Pathological review of late cerebral radionecrosis. Brain Tumor Pathol. 2008;25(2):51–8. doi:10.1007/s10014-008-0233-9. A detailed pathological characterization of radiation necrosis in the brain.
Kureshi SA, Hofman FM, Schneider JH, Chin LS, Apuzzo ML, Hinton DR. Cytokine expression in radiation-induced delayed cerebral injury. Neurosurgery. 1994;35(5):822–9. discussion 9–30.
Hayakawa K, Borchardt PE, Sakuma S, Ijichi A, Niibe H, Tofilon PJ. Microglial cytokine gene induction after irradiation is affected by morphologic differentiation. Radiat Med. 1997;15(6):405–10.
Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23(5):1011–27. doi:10.1200/JCO.2005.06.081.
Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8(8):579–91. doi:10.1038/nrc2403.
Wu Y, Hooper AT, Zhong Z, Witte L, Bohlen P, Rafii S, et al. The vascular endothelial growth factor receptor (VEGFR-1) supports growth and survival of human breast carcinoma. Int J Cancer. 2006;119(7):1519–29. doi:10.1002/ijc.21865.
Curiel TJ, Cheng P, Mottram P, Alvarez X, Moons L, Evdemon-Hogan M, et al. Dendritic cell subsets differentially regulate angiogenesis in human ovarian cancer. Cancer Res. 2004;64(16):5535–8. doi:10.1158/0008-5472.
Proescholdt MA, Heiss JD, Walbridge S, Muhlhauser J, Capogrossi MC, Oldfield EH, et al. Vascular endothelial growth factor (VEGF) modulates vascular permeability and inflammation in rat brain. J Neuropathol Exp Neurol. 1999;58(6):613–27.
van Bruggen N, Thibodeaux H, Palmer JT, Lee WP, Fu L, Cairns B, et al. VEGF antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. J Clin Invest. 1999;104(11):1613–20. doi:10.1172/JCI8218.
Wang W, Merrill MJ, Borchardt RT. Vascular endothelial growth factor affects permeability of brain microvessel endothelial cells in vitro. Am J Physiol. 1996;271(6 Pt 1):C1973–80.
Li YQ, Ballinger JR, Nordal RA, Su ZF, Wong CS. Hypoxia in radiation-induced blood-spinal cord barrier breakdown. Cancer Res. 2001;61(8):3348–54.
Dibbens JA, Miller DL, Damert A, Risau W, Vadas MA, Goodall GJ. Hypoxic regulation of vascular endothelial growth factor mRNA stability requires the cooperation of multiple RNA elements. Mol Biol Cell. 1999;10(4):907–19.
Stacker SA, Vitali A, Caesar C, Domagala T, Groenen LC, Nice E, et al. A mutant form of vascular endothelial growth factor (VEGF) that lacks VEGF receptor-2 activation retains the ability to induce vascular permeability. J Biol Chem. 1999;274(49):34884–92.
Fischer S, Clauss M, Wiesnet M, Renz D, Schaper W, Karliczek GF. Hypoxia induces permeability in brain microvessel endothelial cells via VEGF and NO. Am J Physiol. 1999;276(4 Pt 1):C812–20.
Wang W, Dentler WL, Borchardt RT. VEGF increases BMEC monolayer permeability by affecting occludin expression and tight junction assembly. Am J Physiol Heart Circ Physiol. 2001;280(1):H434–40.
Chuba PJ, Aronin P, Bhambhani K, Eichenhorn M, Zamarano L, Cianci P, et al. Hyperbaric oxygen therapy for radiation-induced brain injury in children. Cancer. 1997;80(10):2005–12. doi:10.1002/(SICI)1097-0142(19971115)80:10<2005.
Hart GB, Mainous EG. The treatment of radiation necrosis with hyperbaric oxygen (OHP). Cancer. 1976;37(6):2580–5.
Kohshi K, Imada H, Nomoto S, Yamaguchi R, Abe H, Yamamoto H. Successful treatment of radiation-induced brain necrosis by hyperbaric oxygen therapy. J Neurol Sci. 2003;209(1–2):115–7.
Leber KA, Eder HG, Kovac H, Anegg U, Pendl G. Treatment of cerebral radionecrosis by hyperbaric oxygen therapy. Stereotact Funct Neurosurg. 1998;70 Suppl 1:229–36.
Levin VA, Leibel SA, Gutin PH. Neoplasms of the central nervous system. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: principles & practice of oncology. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2001. p. 2100–60.
Glantz MJ, Burger PC, Friedman AH, Radtke RA, Massey EW, Schold Jr SC. Treatment of radiation-induced nervous system injury with heparin and warfarin. Neurology. 1994;44(11):2020–7.
Khan RB, Krasin MJ, Kasow K, Leung W. Cyclooxygenase-2 inhibition to treat radiation-induced brain necrosis and edema. J Pediatr Hematol Oncol. 2004;26(4):253–5.
• Gonzalez J, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67(2):323–6. doi:10.1016/j.ijrobp. 2006.10.010. First report of the utility of bevacizumab for radiation necrosis.
Torcuator R, Zuniga R, Mohan YS, Rock J, Doyle T, Anderson J, et al. Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol. 2009;94(1):63–8. doi:10.1007/s11060-009-9801-z.
Wong ET, Huberman M, Lu XQ, Mahadevan A. Bevacizumab reverses cerebral radiation necrosis. J Clin Oncol. 2008;26(34):5649–50. doi:10.1200/JCO.2008.19.1866.
Peak SJ, Levin VA. Role of bevacizumab therapy in the management of glioblastoma. Canc Manag Res. 2010;2:97–104.
•• Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(5):1487–95. doi:10.1016/j.ijrobp. 2009.12.061. The only randomized trial assessing the utility of bevacizumab for radiation necrosis.
Disclosure
Conflicts of interest: J. Fink: none; D. Born: none; M.C. Chamberlain: has been a consultant for and has received payment for development of educational presentations including service on speakers’ bureaus for Genentech/Roche.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fink, J., Born, D. & Chamberlain, M.C. Radiation Necrosis: Relevance with Respect to Treatment of Primary and Secondary Brain Tumors. Curr Neurol Neurosci Rep 12, 276–285 (2012). https://doi.org/10.1007/s11910-012-0258-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-012-0258-7