Abstract
Purpose of review
Two large-scale controlled clinical trials have provided Class I evidence for the benefit of deep brain stimulation (DBS) as a therapy for refractory epilepsy. However, the efficacy has been variable, with some patients not achieving any improvement in their seizure control. This disparity could be the result of suboptimal stimulation parameters/electrodes or alternatively a difference in the type of seizures being treated. This review presents the most recent clinical results with a focus on two major targets for DBS, the anterior nucleus of the thalamus (ANT) and the hippocampus. We detail the etiologies where DBS might work best, and provide evidence for the use of recorded neural responses for the optimization of stimulation parameters and closed-loop control of devices.
Recent findings
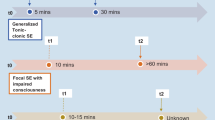
Stimulation of the hippocampus may work well for both focal and generalized seizures, whereas ANT stimulation may be best for focal seizures only. Studies have demonstrated that changes in stimulation-evoked response shape can be used as a biomarker for stimulation efficacy. Furthermore, new biomarkers have been identified that could be used for closed-loop stimulation.
Summary
Improvements in patient screening and stimulation optimization are needed for patients to achieve optimal seizure control. Furthermore, therapy should be adjusted to suit individual patient needs. Recording evoked responses during the application of DBS could be used to measure the effectiveness of DBS and titrate stimulation as needed.


Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mitchell RJ, Herkes G, Nikpour A, Bleasel A, Shih P, Vagholkar S, et al. Examining health service utilization, hospital treatment cost, and mortality of individuals with epilepsy and status epilepticus in New South Wales, Australia 2012–2016. Epilepsy Behav. 2018;79:9–16.
Johnson EK, Jones JE, Seidenberg M, Hermann BP. The relative impact of anxiety, depression, and clinical seizure features on health-related quality of life in epilepsy. Epilepsia. 2004;45(5):544–50.
Schmidt D. The clinical impact of new antiepileptic drugs after a decade of use in epilepsy. Epilepsy Res. 2002;00:1–12.
Miocinovic S, Somayajula S, Chitnis S, Vitek J. History, applications, and mechanisms of deep brain stimulation. JAMA Neurol. 2013;70(2):163–71.
Zhong X-L, Yu J-T, Zhang Q, Wang N-D, Tan L. Deep brain stimulation for epilepsy in clinical practice and in animal models. Brain Res Bull. 2011;85:81–8.
Soss J, Heck C, Murray D, Markovic D, Oviedo S, Corrale-Leyva G, et al. A prospective long-term study of external trigeminal nerve stimulation for drug-resistant epilepsy. Epilepsy Behav. 2015;42:44–7.
Papez JW. A proposed mechanism of emotion. Arch Neurol Psychiatr. 1937;38(4):725–43.
Purves D. Neuroscience. 3rd ed. Sunderland, MA: Sinauer Associates; 2004.
Gliebus GP. Memory dysfunction. Behav Neurol Psychiatry. 2018;24(3):727–44.
Shah A, Jhawar SS, Goel A. Analysis of the anatomy of the Papez circuit and adjoining limbic system by fiber dissection techniques. J Clin Neurosci. 2012;19:289–98.
Vertes R, Albo Z, Di Prisco GV. Theta-rhythmically firing neurons in the anterior thalamus: implications for mnemonic functions of Papez’s circuit. Neuroscience. 2001;104(3):619–25.
Lee K, Meador K, Park YD, King D, Murro AM, Pillai J, et al. Pathophysiology of altered consciousness during seizures: subtraction SPECT study. Neurology. 2002;59(6):841–6.
Child ND, Benarroch EE. Anterior nucleus of the thalamus: functional organization and clinical implications. Neurology. 2013;81:1869–76.
Decarli C, Hatta J, Fazilat S, Fazilat S, Gaillard W, Theodore WH. Extratemporal atrophy in patients with complex partial seizures of left temporal origin. Ann Neurol. 1998;43(1):41–5.
Bertram EH, Scott C. The pathological substrate of limbic epilepsy: neuronal loss in the medial dorsal thalamic nucleus as the consistent change. Epilepsia. 2000;41:S3–8.
Burneo JG, Bilir E, Faught E, Morawetz R, Knowlton RC, Martin R, et al. Significance of fornix atrophy in temporal lobe epilepsy surgery outcome. Arch Neurol. 2003;60:1238–42.
Zumsteg D, Lozano AM, Wieser HG, Wennberg RA. Cortical activation with deep brain stimulation of the anterior thalamus for epilepsy. Clin Neurophysiol. 2006;117:192–207.
Mirski MA. Unraveling the neuroanatomy of epilepsy. Am J Neuroradiol. 1993;14:1336–42.
Han CL, Hu W, Stead M, Zhang T, Zhang JG, Worrell GA, et al. Electrical stimulation of hippocampus for the treatment of refractory temporal lobe epilepsy. Brain Res Bull. 2014;109:13–21.
O’Keefe J. J. Dostrovsky, and J.D. J. O’Keefe, Short Communications The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–5.
Olton DS, Becker JT, Handelmann GE. Hippocampus, space, and memory. Behav Brain Sci. 1979;2:313–22.
Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–8.
Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–9.
Witter MP. The perforant path: projections from the entorhinal cortex to the dentate gyrus. Prog Brain Res. 2007;163:43–61.
Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–8.
Sullivan D, Csicsvari J, Mizuseki K, Montgomery S, Diba K. Relationships between hippocampal sharp waves, ripples, and fast gamma oscillation: influence of dentate and entorhinal cortical activity. 2011;31:8605–16.
Buzsáki G. L. Lai-Wo S., and C.H. Vanderwolf, Cellular bases of hippocampal EEG in the behaving rat. Brain Res Rev. 1983;6:139–71.
Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26.
King D, Spencer SS, McCarthy G, Luby M, Spencer DD. Bilateral hippocampal atrophy in medial temporal lobe epilepsy. Epilepsia. 1995;36:905–10.
Cohen I, Navarro V. On the origin of interictal activity in human temporal lobe epilepsy in vitro. 2002;298:1418–21.
Wenzel M, Hamm JP, Peterka DS, Yuste R. Reliable and elastic propagation of cortical seizures In Vivo. Cell Rep. 2017;19:2681–93.
de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495:387–95.
During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet. 1993;341:1607–10.
Spencer SS, Spencer DD. Entorhinal-hippocampal interactions in medial temporal lobe epilepsy. Epilepsia. 1994;35(4):721–7.
Pinault D, Vergnes M, Marescaux C. Medium-voltage 5–9-Hz oscillations give rise to spike-and-wave discharges in a genetic model of absence epilepsy: in vivo dual extracellular recording of thalamic relay and reticular neurons. Neuroscience. 2001;105(1):181–201.
Williamson P, Thadani V, Darcey T, Spencer D, Spencer S, Mattson R. Occipital lobe epilepsy: clinical characteristics, seizure spread patterns, and results of surgery. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society. 1992;31(1):3–13.
Salanova V, Andermann F, Oliver A, Rasmussen T, Quesney L. Occipital lobe epilepsy: electroclinical manifestations, electrocorticography, cortical stimulation and outcome in 42 patients treated between 1930 and 1991: surgery of occipital lobe epilepsy. Brain. 1992;115(6):1655–80.
Gloor P, Olivier A, Quesney LF, Andermann F, Horowitz S. The role of the limbic system in experiential phenomena of temporal lobe epilepsy. Ann Neurol. 1982;12(2):129–44.
Bonini F, McGonigal A, Trébuchon A, Gavaret M, Bartolomei F, Giusiano B, et al. Frontal lobe seizures: from clinical semiology to localization. Epilepsia. 2014;55(2):264–77.
Shulman MB. The frontal lobes, epilepsy, and behavior. Epilepsy Behav. 2000;1(6):384–95.
Aggleton JP, O’Mara SM, Vann SD, Wright NF, Tsanov M, Erichsen JT. Hippocampal–anterior thalamic pathways for memory: uncovering a network of direct and indirect actions. Eur J Neurosci. 2010;31(12):2292–307.
Mirski MA, Tsai YC, Rossell LA, Thakor NV, Sherman DL. Anterior thalamic mediation of experimental seizures: selective EEG spectral coherence. Epilepsia. 2003;44(3):355–65.
Kusske JA, Ojemann GA, Ward AA Jr. Effects of lesions in ventral anterior thalamus on experimental focal epilepsy. Exp Neurol. 1972;34(2):279–90.
Mirski MA, Ferrendelli JA. Interruption of the mammillothalamic tract prevents seizures in guinea pigs. Science. 1984;226(4670):72–4.
Hamani C, Ewerton FI, Bonilha SM, Ballester G, Mello LE, Lozano AM. Bilateral anterior thalamic nucleus lesions and high-frequency stimulation are protective against pilocarpine-induced seizures and status epilepticus. Neurosurgery. 2004;54(1):191–7.
Mirski MA, Rossell LA, Terry JB, Fisher RS. Anticonvulsant effect of anterior thalamic high frequency electrical stimulation in the rat. Epilepsy Res. 1997;28:89–100.
Velasco AL, Velasco M, Velasco F, Menes D, Gordon F, Rocha L, et al. Subacute and chronic electrical stimulation of the hippocampus on intractable temporal lobe seizures: preliminary report. Arch Med Res. 2000;31:316–28.
Cuéllar-Herrera M, Velasco M, Velasco F, Velasco AL, Jiménez F, Orozco S, et al. Evaluation of GABA system and cell damage in parahippocampus of patients with temporal lobe epilepsy showing antiepileptic effects after subacute electrical stimulation. in. Epilepsia. 2004.
Luna-Munguia H, Orozco-Suarez S, Rocha L. Effects of high frequency electrical stimulation and R-verapamil on seizure susceptibility and glutamate and GABA release in a model of phenytoin-resistant seizures. Neuropharmacology. 2011;61:807–14.
Bondallaz P, Boëx C, Rossetti AO, Foletti G, Spinelli L, Vulliemoz S, et al. Electrode location and clinical outcome in hippocampal electrical stimulation for mesial temporal lobe epilepsy. Seizure. 2013;22(5):390–5.
Vann SD, Aggleton JP. The mammillary bodies: two memory systems in one? Nat Rev Neurosci. 2004;5(1):35.
Graber KD, Fisher RS. Deep brain stimulation for epilepsy: animal models, in Jasper’s basic mechanisms of epilepsies, J. Noebels, M. Avoli, M. Rogawski, R. Olsen, and V. Delgado-Escueta, Editors. 2012. Bethesda.
Avanzini G, Panzica F, De Curtis M. The role of the thalamus in vigilance and epileptogenic mechanisms. Clin Neurophysiol. 2000;111(2):S19–26.
Upton ARM, Amin I, Garnett S, Springman M, Nahmias C, Cooper IS. Evoked metabolic responses in the limbic-striate system produced by stimulation of anterior thalamic nucleus in man. Pacing Clin Electrophysiol. 1987;10(1):217–25.
Li MCH, Cook MJ. Deep brain stimulation for drug-resistant epilepsy. Epilepsia. 2018;59:273–90.
Herrman H, Egge A, Konglund AE, Ramm-Pettersen J, Dietrichs E, Taubøll E. Anterior thalamic deep brain stimulation in refractory epilepsy: a randomized, double-blinded study. Acta Neurol Scand. 2019;139:294–304. Second controlled trial of deep brain stimulation in the ANT. The trial came to an early conclusion due to poor responder rates and in some cases a possible worsening of patient condition. Importantly, electrode positions and stimulation parameters were not specifically selected for individual patient needs.
Kim H, Hur J, Kim H, Park K, Kim S, Hwang T. Modification of electrophysiological activity pattern after anterior thalamic deep brains stimulation for intractable epilepsy: report of 3 cases. Journal of Neurosurgery. 2016;126(6):2028–35 Demonstrates widespread effects of ANT DBS over cortical networks, potentially influencing synchronization over broad areas of brain.
Järvenpää S, Peltola J, Rainesalo S, Leinonen E, Lehtimäki K, Järventausta K. Reversible psychiatric adverse effects related to deep brain stimulation of the anterior thalamus in patients with refractory epilepsy. Epilepsy Behav. 2018;88:373–9 Psychiatric side effects of deep brain stimulation of the ANT can be reversed by changing the stimulating electrodes or stimulation parameters.
Järvenpää S, Rosti-Otajärvi E, Rainesalo S, Laukkanen L, Lehtimäki K, Peltola J. Executive functions may predict outcome in deep brain stimulation of anterior nucleus of thalamus for treatment of refractory epilepsy. Front Neurol. 2018;9:1–8.
Lee C-Y, Lim S-N, Wu T, Lee S-T. Successful treatment of refractory status epilepticus using anterior thalamic nuclei deep brain stimulation. World Neurosurg. 2017;99:14–8.
Kim SH, Lim SC, Kim J, Son B-C, Lee KJ, Shon Y-M. Long-term follow-up of anterior thalamic deep brain stimulation in epilepsy: a 11-year, single center experience. Seizure. 2017;52:154–61.
Guan Y, Chen S, Zhang Y, Liu C, Gu J, Zhao M, et al. The seizure and cognitive outcome of anterior thalamic nucleus deep brain stimulation for patients with intractable epilepsy. Neuropsychiatry. 2017;5(1):04–9.
Koeppen JA, Nahravani F, Kramer M, Voges BR, House PM, Gulberti A, et al. Electrical stimulation of the anterior thalamus for epilepsy: clinical outcome and analysis of efficient target. Neuromodulation. 2018; Electrode selection and the positioning of electrodes for stimulation in the ANT are important as they may determine the degree of reduction in seizure frequency.
Andrade D, Zumsteg D, Hamani C, Hodaie M, Sarkissian S, Lozano A, et al. Long-term follow-up of patients with thalamic deep brain stimulation for epilepsy From Divisions of Neurology. 2006;66(10):1571–3.
Lim SN, Lee ST, Tsai YT, Chen IA, Tu PH, Chen JL, et al. Electrical stimulation of the anterior nucleus of the thalamus for intractable epilepsy: a long-term follow-up study. Epilepsia. 2007;48:342–7.
Hodaie M, Wennberg RA, Dostrovsky JO, Lozano AM. Chronic anterior thalamus stimulation for intractable epilepsy. Epilepsia. 2002;43:603–8.
Kerrigan JF, Litt B, Fisher RS, Cranstoun S, French Ja, Blum DE, et al. Electrical stimulation of the anterior nucleus of the thalamus for the treatment of intractable epilepsy. Epilepsia. 2004;45:346–54.
Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908.
Salanova V, Witt T, Worth R, Henry TR, Gross RE, Nazzaro JM, et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84:1017–25.
Schulze-Bonhage A. Brain stimulation as a neuromodulatory epilepsy therapy. Seizure. 2017;44:169–75.
Keller A, Arissian K, Asanuma H. Synaptic proliferation in the motor cortex of adult cats after long-term thalamic stimulation. J Neurophysiol. 1992;68:295–308.
Miranda MF, Hamani C, De Almeida A-CG, Amorim BO, Macedo CE, José M, et al. Role of adenosine in the antiepileptic effects of deep brain stimulation. Cell Neurosci. 2014;8(312):1–6.
Mirski MA, Ziai WC, Chiang J, Hinich M, Sherman D. Anticonvulsant serotonergic and deep brain stimulation in anterior thalamus. Seizure. 2009;18(1):64–70.
Yu T, Wang X, Li Y, Zhang G, Worrell G, Chauvel P, et al. High-frequency stimulation of anterior nucleus of thalamus desynchronizes epileptic network in humans. Brain. 2018;141:2631–43.
Stypulkowski P, Stanslaski S, Denison T, Giftakis J. Chronic evaluation of a clinical system for deep brain stimulation and recording of neural network activity. Sterotact Funct Neurosurg. 2013;91:220–32.
Stypulkowski P, Stanslaski S, Jensen R, Denison T, Giftakis J. Brain stimulation for epilepsy-local and remote modulation of network excitability. Brain Stimul. 2014;7:350–8.
Covolan L, Almeida A, Amorim BO, Cavarsan C, Miranda MF, Aarao M, et al. Effects of anterior thalamic nucleus deep brain stimulation in chronic epileptic rats. PLoS One. 2014;9:e97618.
Velasco AL, Velasco F, Velasco M, Trejo D, Castro G, Carrillo-Ruiz JD. Electrical stimulation of the hippocampal epileptic foci for seizure control: a double-blind, long-term follow-up study. Epilepsia. 2007;48:1895–903.
McLachlan RS, Pigott S, Tellez-Zenteno JF, Wiebe S, Parrent A. Bilateral hippocampal stimulation for intractable temporal lobe epilepsy: impact on seizures and memory. Epilepsia. 2010;51(2):304–7.
Tellez-Zenteno JF, McLachlan RS, Parrent A, Kubu CS, Wiebe S. Hippocampal electrical stimulation in mesial temporal lobe epilepsy. Neurology. 2006;66:1490–4.
Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295–304.
Osorio I, Frei MG, Sunderam S, Giftakis J, Bhavaraju NC, Schaffner SF, et al. Automated seizure abatement in humans using electrical stimulation. Ann Neurol. 2005;57:258–68.
Morrell MJ, Halpern C. Responsive direct brain stimulation for epilepsy. Neurosurg Clin. 2016;27(1):111–21.
Heck CN, King-Stephens D, Massey AD, Nair DR, Jobst BC, Barkley GL, et al. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia. 2014;55(3):432–41.
Osorio I, Overman J, Giftakis J, Wilkinson SB. High frequency thalamic stimulation for inoperable mesial temporal epilepsy. Epilepsia. 2007;48(8):1561–71.
Vonck K, Boon P, Achten E, De Reuck J, Caemaert J. Long-term amygdalohippocampal stimulation for refractory temporal lobe epilepsy. Ann Neurol. 2002;52:556–65.
Boon P, Vonck K, De Herdt V, Van Dycke A, Goethals M, Goossens L, et al. Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia. 2007;48:1551–60.
Min B, Guoming L, Jian Z. Treatment of mesial temporal lobe epilepsy with amygdalohippocampal stimulation: a case series and review of the literature. Exp Ther Med. 2013;5(4):1264–8.
Cook MJ, O’Brien TJ, Berkovic SF, Murphy M, Morokoff A, Fabinyi G, et al. Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: a first-in-man study. Lancet Neurol. 2013;12:563–71.
Miatton M, Van Roost D, Thiery E, Carrette E, Van Dycke A, Vonck K, et al. The cognitive effects of amygdalohippocampal deep brain stimulation in patients with temporal lobe epilepsy. Epilepsy Behav. 2011;22:759–64.
Voges BR, Schmitt FC, Hamel W, House PM, Kluge C, Moll CKE, et al. Deep brain stimulation of anterior nucleus thalami disrupts sleep in epilepsy patients. Epilepsia. 2015;56:e99–e103.
Stypulkowski P, Giftakis J, Billstrom T. Development of a large animal model for investigation of deep brain stimulation for epilepsy. Stereotact Funct Neurosurg. 2011;89:111–22.
Van Gompel J, Klassen B, Worrell G, Lee K, Shin C, Zhao C, et al. Anterior nuclear deep brain stimulation guided by concordant hippocampal recording. Neurosurg Focus. 2015;38(6):E9.
Zhang S-H, Sun H-L, Fang Q, Zhong K, Wu D-C, Wang S, et al. Low-frequency stimulation of the hippocampal CA3 subfield is anti-epileptogenic and anti-ictogenic in rat amygdaloid kindling model of epilepsy. Neurosci Lett. 2009;455(1):51–5.
Goodman JH, Berger RE, Tcheng TK. Preemptive low-frequency stimulation decreases the incidence of amygdala-kindled seizures. Epilepsia. 2005;46:1–7.
Weiss S, Li X-L, Rosen JB, Li H, Heynen T, Post RM. Quenching: inhibition of development and expression of amygdala kindled seizures with low frequency stimulation. Neuroreport. 1995;6(16):2171–6.
Zhong K, Wu D-C, Jin M-M, Xu Z-H, Wang Y, Hou W-W, et al. Wide therapeutic time-window of low-frequency stimulation at the subiculum for temporal lobe epilepsy treatment in rats. Neurobiol Dis. 2012;48(1):20–6.
Tang Y, Durand DM. A novel electrical stimulation paradigm for the suppression of epileptiform activity in an in vivo model of mesial temporal lobe status epilepticus. Int J Neural Syst. 2012;22(03):1250006.
Wyckhuys T, Raedt R, Vonck K, Wadman W, Boon P. Comparison of hippocampal deep brain stimulation with high (130 Hz) and low frequency (5 Hz) on afterdischarges in kindled rats. Epilepsy Res. 2010;88:239–46.
Boëx C, Vulliémoz S, Spinelli L, Pollo C, Seeck M. High and low frequency electrical stimulation in non-lesional temporal lobe epilepsy. Seizure. 2007;16(8):664–9.
Lee K, Shon Y, Cho C. Long-term outcome of anterior thalamic nucleus stimulation for intractable epilepsy. Stereotact Funct Neurosurg. 2012;90:379–85.
Tyrand R, Seeck M, Spinelli L, Pralong E, Vulliémoz S, Foletti G, et al. Effects of amygdala–hippocampal stimulation on interictal epileptic discharges. Epilepsy Res. 2012;99(1–2):87–93.
Takebayashi S, Hashizume K, Tanaka M. The effect of electrical stimulation and lesioning of the anterior thalamic nucleus on kainic acid-induced focal cortical seizure status in rats. Epilepsia. 2007;48(2):348–58.
Koubeissi MZ, Kahriman E, Syed TU, Miller J, Durand DM. Low-frequency electrical stimulation of a fiber tract in temporal lobe epilepsy. Ann Neurol. 2013;74(2):223–31.
Kalitzin S. D. Velis, P. Suffczynski, J. Parra, and F. Lopes Da Silva, Electrical brain-stimulation paradigm for estimating the seizure onset site and the time to ictal transition in temporal lobe epilepsy. Clin Neurophysiol. 2005;116:718–28.
Cota VR, Drabowski BMB, de Oliveira JC, Moraes MFD. The epileptic amygdala: toward the development of a neural prosthesis by temporally coded electrical stimulation. J Neurosci Res. 2016;94(6):463–85.
Karoly PJ, Goldenholz DM, Freestone DR, Moss RE, Grayden DB, Theodore WH, et al. Circadian and circaseptan rhythms in human epilepsy: a retrospective cohort study. Lancet Neurol. 2018;17:977–85 There are regular cycles that exist in seizure occurrence that are highly patient specific.
Gotman J, Marciani M. Electroencephalographic spiking activity, drug levels, and seizure occurrence in epileptic patients. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society. 1985;17(6):597–603.
Baud MO, Kleen JK, Mirro EA, Andrechak JC, King-Stephens D, Chang EF, et al. Multi-day rhythms modulate seizure risk in epilepsy. Nat Commun. 2018;9:1–10.
Karoly PJ, Freestone DR, Boston R, Grayden DB, Himes D, Leyde K, et al. Interictal spikes and epileptic seizures: their relationship and underlying rhythmicity. Brain. 2016;139:1066–78. Epileptiform spikes and seizures can follow different rhythmicities, which are well characterized within individual patients but highly variable between patients.
Chang WC, Kudlacek J, Hlinka J, Chvojka J, Hadrava M, Kumpost V, et al. Loss of neuronal network resilience precedes seizures and determines the ictogenic nature of interictal synaptic perturbations. Nat Neurosci. 2018;21:1742–52. Epileptiform spikes can cause either anti-seizure or pro-seizure effects depending on the dynamical state of the brain.
Seneviratne U, Boston RC, Cook M, D’Souza W. Temporal patterns of epileptiform discharges in genetic generalized epilepsies. Epilepsy Behav. 2016;64:18–25.
Baud MO, Rao VR. Gauging seizure risk. Neurology. 2018;91:967–73.
Stevens JR, Kodama H, Lonsbury B, Mills L. Ultradian characteristics of spontaneous seizures discharges recorded by radio telemetry in man. Electroencephalogr Clin Neurophysiol. 1971;31:313–25.
Vonck K, Sprengers M, Carrette E, Dauwe I, Miatton M, Meurs A, et al. A decade of experience with deep brain stimulation for patients with refractory medial temporal lobe epilepsy. Int J Neural Syst. 2012;23:1250034.
Bergey GK, Morrell MJ, Mizrahi EM, Goldman A, King-Stephens D, Nair D, et al. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology. 2015;84(8):810–7.
Nune G, Desai SA, Razavi B, Agostini MA, Bergey GK, Herekar AA, et al. Treatment of drug-resistant epilepsy in patients with periventricular nodular heterotopia using RNS® System: efficacy and description of chronic electrophysiological recordings. Clin Neurophysiol. 2019;130:1196–207 Responsive neurostimulation in drug-resistant patients with periventricular nodular heterotopia caused a mean reduction of 85.7% in seizure frequency with very high responder rates.
Milanowski P, Suffczynski P. Seizures start without common signatures of critical transition. Int J Neural Syst. 2016;26:1650053.
Arviv O, Medvedovsky M, Sheintuch L, Goldstein A, Shriki O. Deviations from critical dynamics in interictal epileptiform activity. J Neurosci. 2016;36:12276–12,292.
Kramer MA, Truccolo W, Eden UT, Lepage KQ, Hochberg LR, Eskandar EN, et al. Human seizures self-terminate across spatial scales via a critical transition. Proc Natl Acad Sci. 2012;109:21116–21,121.
Jiruska P, de Curtis M, Jefferys JGR, Schevon CA, Schiff SJ, Schindler K. Synchronization and desynchronization in epilepsy: controversies and hypotheses. J Physiol. 2013;591:787–97.
Meisel C, Kuehn C. Scaling effects and spatio-temporal multilevel dynamics in epileptic seizures. PloS one. 2012;7:e30371.
Cook MJ, Varsavsky A, Himes D, Leyde K, Berkovic S, O’Brien T, et al. The dynamics of the epileptic brain reveal long memory processes. Front Neurol. 2014;5:217.
Meisel C, Schulze-Bonhage A, Freestone D, Cook MJ, Achermann P, Plenz D. Intrinsic excitability measures track antiepileptic drug action and uncover increasing/decreasing excitability over the wake/sleep cycle. Proc Natl Acad Sci. 2015;112:14,694–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Katrina L. Dell and Mark J. Cook each declare no potential conflicts of interest. Matias I. Maturana reports grants from National Health and Medical Research—GNT1130468 and grants from Melbourne Neuroscience Institute Fellowship, during the conduct of the study.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Epilepsy
Rights and permissions
About this article
Cite this article
Dell, K.L., Cook, M.J. & Maturana, M.I. Deep Brain Stimulation for Epilepsy: Biomarkers for Optimization. Curr Treat Options Neurol 21, 47 (2019). https://doi.org/10.1007/s11940-019-0590-1
Published:
DOI: https://doi.org/10.1007/s11940-019-0590-1