Abstract
Drowning is a leading cause of accidental death. Survivors may sustain severe neurologic morbidity. There is negligible research specific to brain injury in drowning making current clinical management non-specific to this disorder. This review represents an evidence-based consensus effort to provide recommendations for management and investigation of the drowning victim. Epidemiology, brain-oriented prehospital and intensive care, therapeutic hypothermia, neuroimaging/monitoring, biomarkers, and neuroresuscitative pharmacology are addressed. When cardiac arrest is present, chest compressions with rescue breathing are recommended due to the asphyxial insult. In the comatose patient with restoration of spontaneous circulation, hypoxemia and hyperoxemia should be avoided, hyperthermia treated, and induced hypothermia (32–34 °C) considered. Arterial hypotension/hypertension should be recognized and treated. Prevent hypoglycemia and treat hyperglycemia. Treat clinical seizures and consider treating non-convulsive status epilepticus. Serial neurologic examinations should be provided. Brain imaging and serial biomarker measurement may aid prognostication. Continuous electroencephalography and N20 somatosensory evoked potential monitoring may be considered. Serial biomarker measurement (e.g., neuron specific enolase) may aid prognostication. There is insufficient evidence to recommend use of any specific brain-oriented neuroresuscitative pharmacologic therapy other than that required to restore and maintain normal physiology. Following initial stabilization, victims should be transferred to centers with expertise in age-specific post-resuscitation neurocritical care. Care should be documented, reviewed, and quality improvement assessment performed. Preclinical research should focus on models of asphyxial cardiac arrest. Clinical research should focus on improved cardiopulmonary resuscitation, re-oxygenation/reperfusion strategies, therapeutic hypothermia, neuroprotection, neurorehabilitation, and consideration of drowning in advances made in treatment of other central nervous system disorders.
Similar content being viewed by others
Introduction
The greatest permanent harm in drowning accidents is to the brain, which has negligible metabolic substrate reserves to subsist upon in the absence of continuous delivery of oxygenated blood. Functional failure begins within seconds after abrupt disruption of circulation at normal body temperature [1]. The brain is sensitive to the timing, duration, and intensity of hypoxia. Irreversible injury develops in the hippocampus, basal ganglia, and cerebral cortex within 4–10 min [2]. Resuscitation at this stage may manifest in memory, movement, and coordination disorders. Only a few additional minutes of hypoxia may result in persistent coma.
Two factors distinguish drowning from cardiogenic cardiac arrest (CA). First, submersion fluids may be cooler than normal brain temperature. Thus, if the drowning process is slow, the body and the brain will cool prior to the arrest. Hypothermia can decelerate neural tissue demise. Second, unlike cardiogenic CA, where there is near-immediate cessation of cerebral blood flow (CBF), submersion results in hypoxic deterioration to asphyxial CA (ACA). Flow continues for some interval, but oxygen delivery is insufficient contributing to injury. Thus, submersion duration may not reliably predict neurologic outcome.
Complicating these matters is delayed neuronal death [3]. Complex cellular responses to hypoxia and re-oxygenation/reperfusion are activated leading to pro-survival and death signals within brain cells. Consequently, immediate neurologic function after drowning rescue does not necessarily predict final outcome. In practical terms, first responders cannot reliably predict outcome at the site of rescue.
The unique sensitivity of brain to hypoxia and the lack of ability to predict outcome at point of rescue creates a challenge, particularly in patients who fail to sustain or recover consciousness. Little can change damage caused by the primary hypoxic event. The best hope is to prevent secondary injury that might exacerbate the initial injury. There is negligible research specific to mechanisms of post-resuscitative brain injury following drowning. The most important defense is to prevent the accident, but drowning still occurs. With modern cardiopulmonary resuscitation (CPR), restoration of spontaneous circulation (ROSC) may be achieved but brain recovery cannot be ensured. The best approach to managing a cerebral insult in survivors of drowning accidents remains undefined. There are no randomized double-blind prospective studies to provide definitive recommendations. Care is largely based on extrapolated knowledge and opinion. This review constitutes an evidence-based consensus effort to provide recommendations for brain-oriented care of drowning victims.
Epidemiology
A new definition of drowning has been established through a consensus procedure: Drowning is the process of experiencing respiratory impairment from submersion/immersion in liquid [4]. The recommended classification scheme is death, morbidity, and no morbidity [4]. With this definition the intention is to put an end to the variety and inconsistency of definitions, particularly the terms dry drowning and near-drowning [5, 6].
The World Health Organization estimates that 388,000 persons die from drowning worldwide annually [7]. In most countries, fatal drowning is among the top three causes of death in children ages 5–14 years, is the most important cause of injury-related death in children younger than four, and drowning accounts for 7 % of all injury-related deaths among all ages globally. The male:female ratio is approximately 4:1 in all ages globally. There are large incidence variations among regions of the world. Low- and middle-income countries account for 97 % of all drownings, of which 49 % occur in China and India alone [7–11].
Data on non-fatal drownings are difficult to obtain. The circumstances of these events can be complex, making it difficult to extract data from hospitals and other sources. Data are often not standardized if reported. A recent Danish study shows a ratio between mortality, morbidity, and no morbidity of 1:0.5:134 [12]. The relationship among these figures would seem highly dependent on the cause of drowning and the victim’s age [13–15]. One to 3 % of all drowning victims admitted to a hospital suffer severe neurological injuries [16]. National death statistics related to drowning contain inaccuracies, often due to poor or deficient coding infrastructure. The latter is often true in low- and middle-income countries, the very countries that experience the highest drowning fatalities. Coding using the tenth revision of the International Classification of Diseases (ICD-10) is strongly recommended [17, 18].
Prehospital Management
Drowning survival depends on a management chain, starting outside the hospital and continuing in-hospital during the post-resuscitation phase. Prehospital care personnel provide opportunity to perform rapid rescue from submersion. Re-establishing ventilation before CA nearly assures no long-term neurological sequelae [19, 20]. If CA occurs, CPR provides the best chance of neurological recovery. Controversy exists over the optimal CPR method. Bystander chest compressions alone may be better than rescue breathing combined with chest compressions (standard CPR) for resuscitating patients with sudden CA [21]. Drowning victims were excluded in this analysis. Drowning victims asphyxiate which leads to CA, so maximizing oxygenation in addition to perfusion during CPR may improve outcome [22, 23]. Two prospective, population-based cohort studies support performing standard CPR for drowning. Rescue breathing and chest compressions [24, 25] and bystanders [26] may play an important role. Thus, it is recommended that drowning victims with no signs of life be provided chest compressions with rescue breathing rather than compression CPR alone.
The best method for ventilating and oxygenating drowning victims has not been established. Supplemental oxygen should be provided to all drowning victims with evidence of hypoxemia or respiratory distress. Continuous or bi-level positive airway pressure may be advantageous if hypoxia is present and spontaneous ventilatory efforts are suboptimal in the conscious patient [27, 28]. Assisted ventilation with a bag-valve-mask should be performed in unconscious patients or those that cannot maintain their airway. Tracheal intubation requires equipment and skill. The decision to intubate at the scene may depend on the expertise of rescuers and first responders. Supraglottic airway devices have been found to not be effective in drowning [29]. Hypoxemia and hyperoxemia should be avoided. Optimal prehospital monitoring includes capnometry if intubation is performed [30] and pulse oximetry [31]. Pulse oximetry may be difficult with hypothermia. Arterial blood gases at hospital admission will best guide ventilatory support.
Arterial hypotension may negatively impact neurological outcome after acute brain injury [32]. Early detection may minimize brain damage. Blood pressure should be assessed at the scene and hypotension should be recognized and treated.
Temperature management is a critical determinant of neurological outcome. Body temperature should be measured as soon as possible after the initial resuscitation. The practice of aggressively warming all drowning victims should be abandoned. Case reports of remarkable neurological recoveries after prolonged CA in ice water drowning suggest a role for therapeutic hypothermia [33–35]. Patients considered candidates for therapeutic hypothermia may benefit from cooling as soon as possible and protocols exist for initiation of cooling by prehospital personnel [36, 37].
Many hospitals have limited experience with brain-directed patient care after ROSC. Prehospital providers may consider direct transport of these patients to facilities with expertise in neurocritical care [38]. Otherwise, transfer of drowning victims who are stabilized at the initial facility to a facility with neuroresuscitation expertise is recommended.
Most drownings occur in low- and middle-income countries. In the absence of advanced resources, key management practices may still be employed including effective CPR, assurance that drowning-induced hypothermia does not explain failure to recover consciousness, avoidance of hyperthermia, and titration of oxygen delivery to prevent hypoxemia and hyperoxemia.
Brain-Oriented Intensive Care Management
Treatment in the intensive care unit (ICU) should be directed toward stabilization of vital functions and prevention of secondary brain injury. This section describes important aspects of ICU treatment for drowning, with emphasis on the brain.
Ventilation and Oxygenation
Submersion in water results in breath holding usually followed by laryngospasm resulting in hypercapnia and hypoxemia. With increasing hypoxemia, laryngospasm abates, increasing risk of water and gastric contents aspiration [22, 39]. Water aspiration may rapidly result in acute lung injury or acute respiratory distress syndrome (ARDS). If so, ventilation/perfusion matching is altered and lung compliance will be low with varying degrees of hypoxemia and hypercapnia.
The incidence of pneumonia associated with submersion is unknown, but findings from case-series suggest rates between 30 and 50 % [40]. The risk is determined by the aspirate (gastric contents/water contamination). Aerobic Gram-negative and Gram-positive bacteria, anaerobes and fungi are common pathogens. Pseudallescheria boydii infections are most frequently reported, which may involve the respiratory tract and other organs, with high mortality rates [41]. The diagnosis of drowning-associated pneumonia may be difficult. Airway cultures may not differentiate between colonization and infection. In patients with fever or systemic signs of infection, antibiotic therapy is advised. One report suggests use of antibiotics found effective in bacteria similar to those found in the body of water where immersion occurred [42]. Routine steroid use should be avoided. Animal and human trials have not identified any clinical benefit.
Lung injury is, in part, the result of washout of surfactant in the lung. There are case reports documenting successful use of exogenous surfactant to treat lung injury associated with pediatric drowning [43–45]. Exogenous surfactant does not reduce mortality or improve pulmonary gas exchange in adult ICU patients [46, 47]. There is one case report of surfactant efficacy in adult drowning [48]. Although unproven, surfactant may be considered as a rescue treatment based on the specific pathophysiology.
No randomized studies have investigated ventilator strategies after drowning. Several animal and case studies support use of positive end-expiratory pressure (PEEP). Based on the underlying pathophysiology, it is reasonable to follow the protective ventilator strategy for ARDS patients. Tidal volumes < 6 mL/kg and sufficient PEEP to avoid extensive lung collapse at end-inspiration are recommended [49], while maintaining plateau pressures as low as possible. Positive pressure ventilation has potential adverse effects on cerebral perfusion. High PEEP levels may decrease cerebral venous outflow, cardiac output, and mean arterial pressure resulting in decreased cerebral perfusion pressure (CPP). In patients with acute brain injury and impaired cerebral autoregulation, PEEP did not change ICP, cerebral perfusion, or oxygenation if adequate blood pressure was maintained [50–52]. PEEP should not increase ICP if ICP exceeds right atrial pressure [53]. The cerebral hemodynamic effects of PEEP may also be related to effects of PEEP on respiratory mechanics [54]. When PEEP is sufficient to induce alveolar hyperinflation, PaCO2 is increased with concomitant ICP increases. When PEEP is limited to cause only alveolar recruitment, no ICP or cerebral perfusion effects occur.
Both hypoxemia and hyperoxemia have been associated with a poor outcome after CA [55, 56], although adverse effects of hyperoxia remain controversial [57, 58]. Current recommendations are titration of inspired oxygen so as to maintain hemoglobin oxygen saturation within 94–98 % once ROSC has occurred and measurement is feasible [59, 60].
Changes in PaCO2 affect CBF. CO2 reactivity is generally preserved in comatose patients treated with therapeutic hypothermia after CA [56]. Arterial hypocapnia decreases bicarbonate resulting in cerebrospinal fluid (CSF) alkalosis, which decreases CBF, cerebral oxygen delivery, and, to a lesser extent, cerebral blood volume. Hypercapnia increases cerebral blood volume thus inducing or aggravating intracranial hypertension. Permissive hypercapnia used as a protective ventilation strategy should not be applied in comatose patients without ICP monitoring. Extracorporeal CO2 removal may be considered in patients with severe ARDS to prevent secondary brain injury. Mechanical ventilation in the prone position may improve arterial and cerebral tissue oxygenation with little adverse effect on ICP [61].
Hemodynamic Support
Hemodynamic failure is rare in resuscitated drowning patients. Most do not aspirate enough fluid to cause clinically relevant changes in blood volume. Drowning in fresh water results in a small and transient increase in blood volume [62, 63]. Aspiration of large quantities of seawater may induce hypovolemia.
There are no studies on the optimal hemodynamic targets in drowning. Since ICP may be elevated [64] and cerebral autoregulation impaired, hemodynamic management is important to ensure cerebral perfusion. Blood pressure should be continuously monitored with an arterial catheter. It is reasonable to target a pressure that is high enough to ensure adequate cerebral perfusion, taking into account for the patient’s normal blood pressure and signs of elevated ICP. If hypovolemia is diagnosed, fluid resuscitation should be initiated rapidly, but monitored. Fluid overload may be adverse. Conservative strategy of fluid management is associated with improved lung function and a shorter duration of mechanical ventilation and ICU stay in patients with acute lung injury [65], although this has not been examined in drowning. Use of inotropes or vasopressors may be necessary. Advanced hemodynamic monitoring may facilitate goal-directed resuscitation.
Seizures
Seizures following hypoxic brain injury are common. Evaluation for seizures allows the potential for treatment and to inform prognosis. Clinical detection of seizures is difficult in the ICU as these patients are frequently sedated and subjected to neuromuscular blockade, thus masking clinical signs. Non-convulsive seizures are common, even in patients who are not paralyzed. Continuous electroencephalographic (EEG) monitoring in critically ill patients revealed a 13 % incidence of seizures; 92 % of those seizures were exclusively non-convulsive [66]. In an unselected population of critically ill children, continuous EEG monitoring detected seizures in 44 % of subjects and 75 % of these patients had purely non-convulsive status epilepticus [67]. Following CA, seizures occur in 47 % of children of which 2/3 had status epilepticus [68]. Myoclonic or EEG status epilepticus has been associated with poor outcome from CA and drowning [69–72]. A small subset of patients with status epilepticus undergoing therapeutic hypothermia may have a good outcome [68, 73–75].
A trial of anti-epileptic drugs is warranted in all patients with clinical seizures. In patients with non-convulsive seizures or non-convulsive status epilepticus, anti-convulsive treatment should be considered, although it is unclear whether treatment impacts outcome. Prophylactic anticonvulsant medications are unproven.
Electrolytes
Serum electrolyte changes after drowning are dependent on the type and amount of fluid aspirated. Clinically relevant electrolyte changes are rare because of the small amount of water that is typically ingested. Even after drowning in the Dead Sea (NaCl concentration in the surface water > 275 g/L) derangements in serum sodium occurred only in a minority of patients [76]. Care must be taken to avoid a hypoosmolar state in patients with a hypoxic encephalopathy, which could promote cerebral edema further aggravate secondary brain damage. Definitive studies of sodium or osmolar management after drowning are lacking.
Glucose Management
Severe illness induces dysglycemia, with potential detrimental effects. Mild hypothermia has an additional effect on glucose homeostasis by decreasing sensitivity to insulin and the amounts of insulin secreted by the pancreas. Both hyperglycemia and hypoglycemia are independent risk factors for increased morbidity and mortality in ICU patients. The injured brain is particularly susceptible to changes in glucose concentrations. Strict glucose control has not proven beneficial in neurocritical care patients and has an unacceptable risk of hypoglycemia when not continuously monitored [77, 78]. The threshold for hypoglycemic injury is likely to be higher in the injured versus normal brain. Even mild hypoglycemia can induce neuroglycopenia. In TBI patients, arterial glucose levels < 108 mg/dL resulted in decreased brain glucose concentrations and increased cerebral glucose uptake [79]. Microdialyzed markers of brain metabolic distress were increased at brain glucose concentrations < 18 mg/dL, reaching the lowest levels at arterial blood and brain glucose levels between 108 and 162 mg/dL. Glucose control in patients after drowning should be focused on avoiding hypoglycemia, hyperglycemia, and wide or rapid fluctuations in glycemic state. There is no target level of blood glucose that has been demonstrated to improve outcome.
Temperature Management
Therapeutic hypothermia has been intensively investigated, the mechanisms of which have been detailed elsewhere [80]. This section will focus on efficacy of induced hypothermia as it may pertain to drowning. A detailed description of how to cool comatose patients for therapeutic hypothermia has been published [81].
Drowning
The use of hypothermia originated for management of the comatose [82] and adult CA patient [83]. In the latter, it was specifically stated that hypothermia should be initiated within 30 min of ROSC. Use of hypothermia in drowning built upon that work, reports of remarkable recovery from cold-water drowning, and an optimistic report on HYPER therapy (diuretics, hyperventilation, deep (30 °C) and sustained hypothermia, barbiturates, and glucocorticoids) [84]. However, therapeutic hypothermia fell out of favor after two small retrospective studies suggested that deep and sustained hypothermia applied in an aggressive resuscitation regimen to patients with absent vital signs on admission increased survival of patients in a persistent vegetative state only [85, 86]. Hypothermia was abandoned until laboratory studies identified the need for only moderate hypothermia to mitigate neuronal injury. Two randomized clinical trials in CA showed improved neurologically intact survival with use of moderate hypothermia [87, 88] allowing reconsideration of efficacy in asphyxial injury.
Asphyxia
All but one study (children [89]) analyzed newborns (Fig. 1). There were two systematic reviews [90, 91], and five randomized clinical trials (RCT) [92–96] showing increased chance of outcome without disabilities with mild hypothermia therapy. The relative risk (RR) for reduction of death or major neurodevelopmental disability varied between 1.35 and 2.43. The first systematic review included 8 RCTs and showed therapeutic hypothermia to be beneficial to term newborns with hypoxic ischemic encephalopathy (HIE) [90]. The second included 6 RCTs and associated therapeutic hypothermia with a highly reproducible reduction in the risk of the combined outcome of mortality or moderate-to-severe neurodevelopmental disability [91]. In 4 RCTs [97–100] and in 1 non-randomized comparative trial [89], no difference was found between hypothermia and normothermia groups. In a feasibility RCT performed in a low-resource setting, survival was decreased with hypothermia therapy [101]. Follow-up work is underway [102].
Cardiac Arrest
Two randomized trials demonstrated improved neurological outcome at hospital discharge or at 6 months after hospital discharge in comatose patients after out-of-hospital ventricular fibrillation (VF) CA [87, 88]. A target temperature of 32–34 °C was maintained for 12–24 h. One small randomized trial of comatose patients after CA with non-shockable rhythms showed a higher rate of neurologic intact survival in patients treated with a cooling cap [103].
A systematic review demonstrated improved neurologic survival from therapeutic hypothermia with a RR of 1.55 (95 % CI 1.22–1.96) compared with standard post-resuscitation care [104]. Fourteen studies of comatose survivors of CA using historical controls found therapeutic hypothermia to improve neurological outcome [105]. This is in contrast to a more recent meta-analysis [106], which reported a low-quality evidence in existing studies due to not aggressively treating hyperthermia in patients randomized to control groups.
Summary
There are no high-level evidence studies of therapeutic hypothermia in drowning. Treatment recommendations are made by extrapolation from studies of asphyxia and CA. First, prevent hyperthermia in comatose victims. If hyperthermia occurs, treat it promptly. Second, consider maintaining a target core temperature of 32–34 °C for 12–72 h. Cooling should be started as soon as possible, and re-warming should be slow, at a rate no faster than 0.5 °C/h.
Pediatric Considerations
For every pediatric drowning death, at least two non-fatal drowning hospitalizations are reported [107]. Pediatric hospitalizations in the USA have decreased over the past 16 years, from 4.7 to 2.4 per 100,000 population [108]. Roughly 80 % of children with a history of drowning are admitted to hospital for at least 1 day, and as many as 20 % of survivors suffer severe and sustained disability [109–111]. Factors that influence outcome include the preexisting medical condition, initial electrocardiographic rhythm, duration of no-flow time, and the quality of the life-supporting therapies provided during and following resuscitation. Higher survival rates in children may be due to healthier baseline health status, improved CBF during CPR because of increased chest compliance, and reserve and tolerance of the young brain to hypoxia/ischemia.
Considerations for pediatric drowning resuscitation involve age-dependent developmental physiology, anatomy, pharmacology, and psychology. The most commonly discussed physiologic consideration is persistence of the diving reflex [112] that occurs with rapid immersion in almost 100 % of babies to the age of 6 months, and persists in 10–15 % of older children and adolescents. This response, which starts immediately upon submersion prevents aspiration of water, re-distributes oxygen to heart and brain, slows cardiac oxygen use, and initiates a hypometabolic state. An intact diving reflex may be associated with improved outcomes in young children, and is accentuated when the “dive” is into ice-cold water [112].
A child’s relatively large head-to-body ratio allows for rapid surface cooling of the brain. Young infants may have an open fontanelle, decreasing risk for increased ICP. Lungs are relatively small, with increased airway resistance and relatively easily depleted surfactant stores. The functional residual capacity is small, with a closing lung capacity that is near functional residual capacity increasing risk of atelectasis without PEEP. Their flat diaphragms provide little anatomic advantage, and lower esophageal sphincter tone compared to adults leads to stomach distension with air and fluid. They generally have healthy hearts and thus tolerate high heart rates and catecholamine infusions. Rapid vascular access, monitoring, and rescue equipment sizing can be challenging.
Children have developmental changes specific to enzyme activity, integrity of the blood–brain barrier, high cerebral oxygen and glucose consumption, and unique patterns of biomarker and inflammatory mediator responses [113, 114] to hypoxic–ischemic brain injury. They have unique patterns of energy utilization, brain dependence on glucose and ketones, and glucose transport [115, 116]. Pharmacokinetics and pharmacodynamics change as volume of distribution (water content) and compartments change, and metabolic enzymes mature. Neuronal plasticity changes over time [117].
Although there are unique features to childhood drowning, there is not yet a unique goal-directed approach that consistently differs from adult brain-oriented ICU principles.
Neuromonitoring
In a survey of 72 patients admitted to the hospital for drowning, 21 % had severe anoxic encephalopathy [118]. Neurologic monitoring following resuscitation from drowning is important for prognosis, titration of therapies, and stratification of patients eligible for interventional trials. In this section, specific focus will be on the use of non-invasive monitoring techniques including neurologic examination, EEG, neuroimaging, somatosensory evoked potentials (SSEP), and invasive ICP monitoring. Induced hypothermia may impact the timing and prognostic reliability of these techniques.
Neurologic Examination
Standard neurologic examination is the most pragmatic tool. Many patients who are awake and interactive on emergency department arrival will survive grossly intact. It is not as clear which patients who have altered mental status or coma will do poorly [119, 120].
Large and unreactive pupils are a hallmark of HIE. It is unclear when the absence of pupillary responsiveness to light predicts poor outcome. Small retrospective drowning studies have shown absence of pupillary response in the emergency department or ICU to be associated with poor neurologic outcome [119–123]. While pupillary response may return within hours of resuscitation [118], an absent pupillary response at 24 h is associated with poor neurologic outcome [70, 124, 125]. This marker may be influenced by therapeutic hypothermia [126].
While drowning victims who are awake on ICU admission do well, patients who are non-responsive within 24 h of resuscitation do not have a uniformly poor outcome [119, 120, 125]. A composite GCS score ≤ 5 on ICU admission after drowning is associated with poor outcome, however, it is only 75 % specific to detect patients with a poor outcome. Some patients with a GCS ≤ 5 on ICU admission may have intact neurologic survival [122, 123].
Admission neurologic examination, alone, is not predictive of outcome. Neurologic examinations should be serially repeated including level of consciousness, pupillary reactivity, brain stem reflexes, and motor function. Drowning victims have recovered motor function as late as 48 h after resuscitation [127, 128]. CA data supports these findings [70, 71, 124, 129, 130]. Following drowning, prediction of poor outcome cannot reliably occur based on absence of pupillary reactivity until 24 h after resuscitation and absence of motor score until 72 h after resuscitation.
Electroencephalography
Continuous EEG can be used for prognostication of brain injury severity, diagnosis, and titration of treatment for seizure activity, or stratification of patients for clinical studies. Trained neurologists usually perform EEG interpretation. In certain settings, real-time quantitative tools can be interpreted by ICU providers [131]. EEG has been used to prognosticate severity of injury following hypoxic ischemic insults and CA. There are little data specific to drowning. Non-standardized terminology makes it challenging to generalize findings. In small retrospective studies of drowned children, EEG progression to burst suppression was associated with poor outcome. The presence of normal sleep wake differentiation was associated with good outcome [69, 132]. Burst suppression, isoelectric patterns, profound background attenuation, and loss of reactivity to stimulation are associated with poor outcomes following CA and hypoxic ischemic injury (Fig. 2) [71, 125, 133–135]. Continuous EEG background with reactivity is re-assuring [71, 135].
Multiple CA studies evaluated the prognostic value of EEG background patterns during therapeutic hypothermia. Hypothermia can impact EEG background activity. The most profound effects are seen at temperatures below the target range of 32–34 °C. A study of 19 pediatric CA patients showed burst suppression or excessive EEG discontinuity to be predictive of seizures [68]. In a larger pediatric study, the presence of continuity without reactivity, discontinuity, burst suppression, or isoelectric background was associated with poor outcome during both hypothermic and normothermic phases of induced hypothermia [136]. Similar to normothermia, lack of EEG reactivity, burst suppression, and isoelectric patterns are associated with poor outcome in adult CA [74, 75, 126, 137]. Patients who demonstrate EEG reactivity with stimulation are likely to have a good outcome [137].
Neuroimaging
Early neuroimaging after drowning allows detection of injury requiring neurosurgical intervention and may aid in prognosticating the severity of brain injury.
Computerized Axial Tomography
A CT may be useful to detect head and neck trauma. An initially normal CT in a comatose patient should not be re-assuring [138, 139]. Comatose pediatric drowning victims who have an initial abnormal head CT or a normal CT with a subsequent abnormal CT have poor outcome [139]. Retrospective studies concluded that if there was no clinical evidence of trauma, CT is not necessary [138, 139]. Despite this, intracranial hemorrhage may be present following CA [140]. Early post-ROSC CT imaging may show loss of gray white matter differentiation and cerebral edema, which are associated with poor CA outcome [141, 142]; sulcal effacement is less reliable [143, 144]. The presence of a reversal sign (a bright cerebellum and dark cortex) is associated with poor outcome [145]. Early loss of gray white matter differentiation may not be improved by hypothermia [146, 147].
Magnetic Resonance Imaging (MRI)
Diffusion weighted imaging (DWI) can detect injury within minutes after ischemia and improves the ability to predict poor outcome [148]. Cortical and deep brain DWI abnormalities are associated with poor outcomes as are lower apparent diffusion coefficients (Fig. 3) [149–151].
Magnetic resonance imaging (MRI) of the brain in two children with divergent outcomes after drowning accidents. a–c A 4-year-old cold-water drowning victim who presented with a core temperature of 23 °C. MRI 4 days after the accident was normal as was the child’s neurological examination. d–f A 13-month-old bathtub-drowning victim who presented with a core temperature of 33 °C. MRI 4 days after the accident showed increased signal and restricted diffusion in the basal ganglia (arrows), particularly in the globus pallidus as well as the thalami. In addition, there are areas of restricted diffusion in the white matter bilaterally and to a lesser degree of the cortex most marked in the occipital and parietal regions (open arrows). This child remained in a minimally conscious state. DWI diffusion-weighted imaging, ADC apparent diffusion coefficient
MRI grading systems have been proposed to prognosticate outcome. An 8-point system in children with hypoxic coma yielded a sensitivity of 96 % for an abnormal MRI observed within the first 3 days after injury to predict poor outcome. However, a normal early MRI was only 50 % specific (predictive) for good outcome. MRIs obtained 4–7 days following injury have a higher predictive value [152]. In drowning, children who had MRI evidence of basal ganglia injury, edema, and cortical abnormalities did poorly [153].
MR spectroscopy, which evaluates the metabolic profile of the brain, best predicts outcome 3–4 days after drowning and CA. When evaluated 1–2 days post-injury, several patients who remained vegetative or died had normal scans. MR spectroscopy sensitivity improved over time and had a lower false negative rate several days after injury [153, 154].
SSEP
SSEP measures an electrical signal in response to a sensory stimulus. N20 SSEP specifically evaluates the response in primary sensory cortical locations. Electrical noise can impact its accuracy. A skilled electrophysiologist must perform and interpret SSEP. Neurologic examination in patients with post-anoxic coma does not reliably predict N20 responses after CPR [155].
Following adult CA, bilateral N20 SSEP absence is sensitive for poor outcome between 24 and 72 h following resuscitation [70, 133]. Although several subjects will regain SSEP later, in one study all the subjects still had poor outcome. While N20 SSEP absence predicts poor outcome, N20 SSEP presence at 24 h does not always predict a good outcome. In pediatric CA, bliateral N20 SSEP absence at 24 h is also highly predictive of poor outcome [125, 156].
ICP
Intracranial hypertension is common after drowning and is strongly associated with a poor outcome [157–159]. Initial cerebral edema is most likely cytotoxic due to energy failure and transit of water into the intracellular compartment. This can contribute to intracranial hypertension and decreased CBF. While CBF improves during the first 24 h after ROSC, cerebral edema worsens as tissue deteriorates [157]. ICP monitoring is invasive with adherent risks. There has been no optimal anatomic site identified for monitoring ICP following drowning. Retrospective review showed that elevated ICP did not correlate with postmortem cerebral edema and that ICP monitoring did not impact drowning outcome [85]. No randomized trials are available for ICP monitoring and treatment in drowning. Pediatric case series have reported disappointing results with patients surviving in a vegetative neurologic state despite adequate ICP and CPP control [157–161]. One small prospective drowning study showed no benefit [162]. Currently, ICP monitoring is not routinely used following drowning. Re-evaluation of ICP-guided therapy in the context of current care is warranted. Modest elevation of the head reduces ICP. The lowest mean ICP is usually found with elevation of the head to 30° [163]. Elevation beyond 30° may adversely affect CPP.
Investigational Neuromonitoring
Continuous monitoring may allow the clinician to titrate therapies. Stratification of injury may also define which patients, based on severity of injury and individual characteristics, may benefit from select therapies. Microdialysis, brain tissue oxygenation monitors, near-infrared spectroscopy, and quantitative EEG transformations may be useful. Xenon CT and arterial spin-labeling MRI may allow intermittent evaluation of brain perfusion. Positron emission CT may allow intermittent evaluation of metabolic function.
Summary
Neurologic examination, EEG, neuroimaging, and SSEP can help prognosticate neurologic outcome after drowning (see Table 1). No single tool can reliably predict outcome prior to 24 h from resuscitation. The combination of these methods may enhance outcome prediction. Therapeutic hypothermia may necessitate re-calibrating the impact of the prognostic values of these tools and clinicians should be cautious in making predictions based on normothermic-derived investigations. Evaluation of established and novel neuromonitoring modalities in the drowning population is necessary.
Biomarkers
Biomarkers are quantifiable biological parameters, often proteins or parts thereof, which may be detected in a fluid compartment of the human body. This fluid compartment is often peripheral blood but can also be urine or CSF. The significance of biomarkers relates to drowning outcome and how they can aid in prognostication. There are few data and no good quality trials on use of biomarkers after drowning. Current understanding is predominantly based on extrapolation from data after CA. This seems relevant since the divider between a good and a poor outcome after drowning, other than submersion duration, often is whether or not the patient suffered CA [86, 164].
Biomarkers may be organ specific or more general, the latter reflecting a non-specific stress response. Drowning results in asphyxia and may lead to CA. Therefore, biomarkers of interest after drowning should be those mirroring an asphyxial insult to the brain, for example neuron-specific enolase (NSE) or S-100B, or an asphyxial insult to the heart (troponin I and T) [165]. Biomarkers of a non-specific stress response include blood glucose, lactate, cytokines, and products of oxidative stress (Table 2). Biomarkers are easy to sample and inexpensive to measure. Drawbacks include a multitude of methods in clinical use and lack of a standard, making it difficult to compare results between centers and to produce guidelines. Anticoagulant therapy or possible herniation may exclude use of CSF.
NSE is the best-studied biomarker and may be used as an adjunct in prognostication after CA [114, 166–168]. However, results from centers differ due to differences in methodology and lack of standard assay procedure [169, 170]. A low or moderately elevated NSE value (< 33 μg/L) in a patient remaining comatose should alert the clinician that a treatable condition might prevail [171]. A high NSE (> 33 μg/L) is often not only a sign of extensive injury but also may be caused by hemolysis, due to NSE in erythrocytes. Caution is warranted and biomarkers, like NSE, should never be the sole basis for a decision on level of care. Only one case report was identified where NSE-levels were presented in a drowning victim with CA [172]. This patient had a good outcome and peaked at 20.7 μg/L, well below the 33 μg/L cut-off, which has been suggested in guidelines [71]. Other brain biomarkers that have been studied after CA without submersion include S-100B [166, 173, 174], GFAP [175], and tau [176].
Regarding biomarkers of a non-specific stress response, an association has been shown between high plasma glucose on admission and a poor drowning outcome [120, 123, 177]. Typically, admission plasma glucose values < 200 mg/dL were associated with better outcome. This is consistent with threshold values reported in other forms of acute brain injury. High blood lactate or low pH on admission may associate with a poor outcome after drowning, but the results are conflicting [13, 178]. Pro-calcitonin is another surrogate marker for CA outcome that has attracted interest [179, 180]. Its use after drowning has not been studied. Novel biomarkers to aid in prognostication after drowning may emerge. Such candidates include non-specific markers of oxidative stress and inflammation and novel, ultrasensitive markers of organ injury such as organ-specific micro-RNAs measured in peripheral blood [181].
A single sample of any biomarker may be false for various reasons. Repeated samples should always be secured, preferably as a timed series to capture trends that may add to their prognostic value [166, 168]. The issue of standardization of analytic procedures and normal values across laboratories must be solved before general recommendations regarding any biomarker can be given.
Summary
There are few data on use of biomarkers to aid in prognostication after drowning. Troponin I, plasma glucose, and possibly blood pH and lactate on admission are associated with outcome but the data is scarce and conflicting. The best-studied biomarker after CA is NSE and its use may be considered after drowning. Induced hypothermia for treatment of comatose survivors may affect the release profile of biomarkers and alter their predictive value. This is under active investigation in CA. Prospective high-quality studies to investigate the prognostic value of biomarkers after drowning are warranted.
Post-hypoxic Pharmacology
Systematic assessment of pharmacologic therapy for treatment of brain injury in drowning is non-existent. This is attributable to numerous factors. Drowning events are sporadic, the frequency of which vary with season, location, and age. Drowning reflects a heterogeneous presentation of anoxic brain insults. The duration of submersion, and thus asphyxia is often unknown. Drowning in cold water may be attributable to submersion alone or instead to hypothermia, which independently causes CA followed by submersion. There is little evidence distinguishing cerebral responses to fresh versus salt water drowning. Drowning may be complicated by TBI, spinal cord injury, drug use, or other factors. Finally, there are no pharmacologic agents with proven efficacy in HIE insults of any etiology that could be directly extended to drowning. These factors, combined, have made studies of pharmacologic efficacy in the drowning population implausible to date.
Given the potentially catastrophic outcome associated with a comatose state after ROSC in drowning, caregivers may consider application of clinically available putatively efficacious pharmacologic interventions, even if sufficient and rigorous evidence is not in hand to justify such therapy. Thus, data from other HIE insults have been considered to determine whether such efforts are appropriate.
Anesthetics
Barbiturates
Evidence that barbiturates substantially suppress cerebral metabolic rate offered hope that deprivation of energy substrate could be offset by decreased energy requirement. Although ATP synthesis was later shown to rapidly recover after restoration of CBF, efforts to use barbiturates for neuroresuscitative purposes continued.
The concept of using barbiturates for treatment of drowning was introduced as a component of HYPER therapy [182]. A retrospective investigation reported outcome advantages [183], but further work was not supportive [85]. Multiple interventions were used simultaneously making it difficult to discern effects of most components of HYPER therapy. However, barbiturates have been specifically investigated. Outcome in pediatric drowning victims treated with HYPER therapy was no different with or without inclusion of the barbiturate component [184]. This parallels data from a prospective randomized trial of barbiturates in adult comatose survivors of CA, where thiopental failed to alter outcome [185, 186], and results of randomized trials of barbiturate loading in severe perinatal asphyxia [187, 188]. Rigorous study indicates no role for barbiturates in drowning.
Lidocaine
Lidocaine, in antiarrhythmic doses, has been reported to decrease cognitive deficits after cardiac surgery [189, 190]. The mechanisms are presumed to be inhibition of cellular ion fluxes and cerebral metabolic suppression [191]. No clinical trials have examined lidocaine neuroresuscitative efficacy in drowning, CA, or asphyxia.
Glutamate Antagonists and Calcium Channel Blockers
Extracellular glutamate is increased in ischemic/anoxic brain leading to toxic increases in intracellular calcium and sodium. Multiple glutamate receptor antagonists have been studied experimentally. None has achieved approval as neuroresuscitative agents. Magnesium and L-type calcium channel blockers are clinically available and have been proposed as having neuroprotective properties by inhibiting calcium entry into neurons, similar to selective glutamate antagonists.
Magnesium
No studies have been conducted in drowning. In perinatal asphyxia, five trials have been reported. EEG and outcome responses were studied in 30 neonates randomized to MgSO4 or 0.9 % NaCl treatment [192]. No effect of magnesium was observed other than decreased blood pressure. This was followed by a trial in neonates randomized to MgSO4 with dopamine or placebo [193]. MgSO4 plus dopamine improved short-term outcome. Forty neonates were randomized to MgSO4 or saline [194]. Short-term neurologic outcome was better with MgSO4. Another trial studied 40 patients followed for 6 months after birth [195]. A pattern of MgSO4 efficacy was present, but not consistent. None of these studies evaluated large populations and mechanisms of action were not addressed. Extension of birth asphyxia to drowning in children and adults is not supported by direct evidence.
There was only one trial of magnesium treatment in adult CA [196]. Three hundred patients were randomized to receive placebo, 2 g MgSO4, or 2 g MgSO4 plus 10 mg diazepam. No adverse events were observed, but there was no benefit from treatment. Magnesium worsened morbidity and increased mortality in a large TBI trial [197]. While the CA study was negative, it has been suggested that a combination of induced hypothermia and magnesium may be efficacious [198]. In summary, there is no evidence that magnesium improves drowning outcome. Although showing some benefit in birth asphyxia, magnesium has no benefit in CA and worsens TBI and thus cannot be recommended.
Calcium Channel Blockers
L-type calcium channel blockers are purported to impede Ca++ entry into neurons rendering them more tolerant to ischemia. One drowning case report was retrieved. A 27-month-old child made a full recovery from a comatose state following drowning and CPR. Verapamil (1 mg) was given creating hemodynamic instability [199]. Hemodynamic instability was also observed in four full-term asphyxiated infants treated with nimodipine [200]. Two clinical trials have been conducted. Out-of-hospital VF CA patients were randomized to nimodipine or placebo with no change in 1-year survival [201]. Similar findings were reported for lidoflazine [202]. These studies ended efforts to further explore calcium channel blockers, which are not recommended in drowning.
Antioxidants
Oxidative stress is a known component of ischemic/asphyxia brain injury. Pharmacologic inhibition of free radical damage and signaling has been found therapeutic in preclinical experimental paradigms. Antioxidants have not been studied in drowning. One randomized trial found no benefit from ascorbic acid and ibuprofen in asphyxiated neonates [203]. There were no articles associated with CA. Allopurinol inhibits xanthine oxidase decreasing superoxide production [204]. Three trials were performed in perinatal HIE [205–207] and subjected to meta-analysis. Insufficient evidence was available to define efficacy [208]. A major allopurinol trial is now underway [209]. No work has been done on modern molecules offering known blood–brain barrier penetration or potent redox activity. To date, there is insufficient evidence that clinically available drugs having anti-oxidant activity will be of benefit in drowning.
Glucocorticoids
Glucocorticoids are hypothesized to stabilize cellular membranes exposed to oxidative stress. Searches retrieved only case reports for glucocorticoid use in drowning [210]. Glucocorticoids were typically given in the context of barbiturate coma and hyperventilation. Specific glucocorticoid efficacy could not be determined. There are no recent reports in drowning representing abandonment of this therapy. For CA, two large retrospective studies were identified with concurrent controls [211, 212]. No benefit from glucocorticoid use was observed. No evidence was found to support glucocorticoid use in drowning.
Erythropoietin
Erythropoietin is clinically available to stimulate erythropoiesis. Erythropoietin receptors are present in brain. Erythropoietin has growth factor, anti-apoptotic, anti-oxidant, and anti-inflammatory properties. Structural modifications of the parent molecule are being developed to preserve neuroprotective properties, without stimulating erythropoiesis. Erythropoietin has not been evaluated in drowning, but has been investigated as a post-ischemic/anoxic therapeutic [213]. CA patients were treated with erythropoietin during chest compression [214]. Neurologic outcome was not assessed. In another study [215], patients were treated with erythropoietin and hypothermia. Erythropoietin had no benefit, but thrombocytosis was increased.
Erythropoietin has been studied in neonatal HIE. Eighteen-month outcomes were assessed in neonates with asphyxia injury [216]. Erythropoietin did not have an effect on primary neurologic outcome end-points. Post hoc analysis of neonates with only moderate asphyxia found benefit, suggesting efficacy only with specific severities of injury. A case–control study on a small population of neonates with birth asphyxia found fewer neurologic and developmental abnormalities at 6 months with erythropoietin [217].
Optimism remains for a clinical role for erythropoietin in CA and birth asphyxia, but efficacy is unproven and adverse events are unmeasured. There are no data specific to drowning. There is insufficient evidence to recommend use of erythropoietin in drowning.
Summary
There have been no systematic pharmacologic studies in drowning outside study of HYPER therapy, which combined numerous interventions and no benefit was observed. Thus, no neuroresuscitative pharmacologic therapy can be recommended. Brain pathobiology following a severe and sustained reduction in critical substrate delivery is complex. Research efforts continue to reveal novel targets for pharmacologic intervention. As new pharmacologic opportunities emerge, investigation in drowning remains a priority.
Animal Experimentation
The principle etiology of the neurological insult in drowning is ACA. Rodent and large animal ACA models offer acute physiology and, in some cases, neuropathological and behavioral outcomes. Both adult and pediatric models are available. There are few drowning models [218]. ACA models show important pathophysiological differences from VF, supporting the need to use ACA models for drowning research. In contrast to other forms of acute brain injury, ACA represents one of the few experimental conditions where translation of a beneficial neuroresuscitative therapy from animals to human has been shown, namely conventional versus compression only CPR in ACA (unlike VF). This was originally shown in a piglet ACA model [219] and predicted results from large-scale CPR studies in children [24, 25]. Similarly, mild hypothermia was successfully translated from neonatal asphyxia sheep models to clinical use [220].
ACA Models that Include Drowning
Animal models have generally not incorporated drowning into an ACA paradigm and those that exist have not been used to study neuroprotection. One exception examined cold-water exposures in canine models and showed benefit of hypothermic protection [218]. The value of intra-arrest hypothermia in cold water was already supported by clinical literature [182, 221]. Given the lack of use of drowning models, the recommendations that can be gleaned from preclinical research are based on ACA studies alone.
Rat ACA Models
There are excellent models that produce ACA in anesthetized adult rats using neuromuscular blockade, which, after a brief interval results in a no-flow state, mimicking the human condition [222–229]. The model incorporates a clinically realistic resuscitation, post-arrest coma, long-term survival (after 6–8 min insults), and characteristic delayed neuronal death in selectively vulnerable regions. It can be used to test behavioral outcomes [222, 228] and molecular tools are available for study of rats. Limitations include a short CPR phase (usually < 1 min) and the inability to perform long-term ICU care. The model is excellent for screening therapies and is inexpensive. Several therapies have shown promise in this model, e.g., mild hypothermia, glucose and insulin, and neurotensin [223, 226–229]. There also are pediatric analogs [230–233], which use post-natal Day 17 rats. Promising results have been obtained with hypothermia and minocycline [231, 233]. Mouse ACA models have not been developed.
Pig ACA Models
Due to their size, pigs better mimic the CPR-related aspects of drowning resuscitation and ICU care is feasible [234–237]. However, neuropathological targets and long-term outcome are not well defined. Behavioral testing is also poorly developed limiting ability to link structure and function in contrast to rodents in which cognitive function can be readily assessed with mazes. There are few molecular tools for use in pigs, although this is improving. Expense is another concern. There are pediatric piglet models [219, 238–245]. More work has been done in pediatric than adult ACA models likely related to the importance of ACA in children. Once again, piglet CPR-related physiology mimics many aspects of the human condition. Use of piglet ACA models to optimize CPR has had excellent success for direct translation to the clinical condition [219, 238–245]. However, neuropathological targets are less defined than in rodent models and behavioral testing of piglets limited. Nevertheless, a number of aspects of cardiovascular and brain development are similar to humans [246]. Thus, there is opportunity for drowning studies in piglet ACA.
Canine ACA Models
Adult canines have been used to characterize ACA physiology, neuropathology, and functional outcome. Direct comparisons to other forms of CA, namely VF, have been made [218, 247–249]. For comparable no-flow durations, despite a shorter time to ROSC after ACA than VF, more severe brain injury is produced by ACA (Fig. 4). Neuronal death in selectively vulnerable regions was seen in both ACA and VF, but severe cortical microinfarcts were seen only after ACA. Histopathological damage scores were twice has high in ACA. Treatment targeting oxidative stress was effective in ACA while a calcium antagonist showed benefit in VF. Thus, therapeutic approaches may need to be different in ACA. ICU care has been applied to these models [218, 247–249]. Efficacy of intra-arrest deep hypothermia has been demonstrated in drowning-induced canine ACA [218]. As with pigs, cognitive outcome testing is limited and studies are expensive. Pediatric canine studies have targeted neonatal asphyxia rather than drowning [250, 251].
Responses to asphyxial versus ventricular fibrillation cardiac arrest. Schematic based on the seminal work of Vaagenes et al. [249] using canine models of asphyxial versus ventricular fibrillation cardiac arrest. Despite identical (10 min) no flow durations in both models, a much more severe neuropathology was observed after asphyxial arrest. This was accompanied by poorer neurological outcome. Notably, the no flow period in asphyxial cardiac arrest was preceded in these studies by a period of ~7 min during which hypoxic perfusion of the brain occurred. The asphyxia scenario is highly germane to the pathobiology of drowning and thus suggests a very challenging and unique condition for the development of novel therapies
Summary
Excellent ACA models, across species and age, have been successfully used to translate therapies to humans. ACA differs importantly from VF with regard to neuropathological damage and possibly response to therapy. There is need for systematic evaluation of putative neuroresuscitative therapies specific to ACA if treatment is to be made available for drowning. There is also a need for better characterization of neuropathology and functional deficits in large animal models. Similarly, there is a need for ACA models that incorporate drowning since secondary insults could complicate neurological outcomes.
Future Research Questions
Key unanswered questions related to the development of optimal neuroresuscitation in drowning are presented below.
-
(1)
How should clinical targets for neuroprotection in drowning be identified? Clinical descriptive and outcome studies are needed [252, 253]. Guidelines for study of drowning have been recommended to standardize definitions, terminology, and data sets [6]. Advanced neuromonitoring, serum biomarkers, and brain imaging should be used to define key targets for neuroprotection, evaluate therapeutic efficacy, and predict outcome [136, 139, 153, 166, 174]. To define therapeutic targets, better use should be made of existing animal ACA models [224, 230, 249], and like clinical studies consider standardization of definitions, terminology, and recommended datasets.
-
(2)
What is the impact of conventional versus compression only CPR on neurological outcome in drowning? Data from adults, children, and laboratory piglet models have demonstrated a more favorable neurological outcome after ACA using conventional rather than compression only CPR [25, 254]. Although one would anticipate similar findings in drowning, data are needed. In addition, how other aspects of basic and advanced life support (e.g., management of arterial oxygen partial pressure, blood glucose, body temperature) influence neurological outcome in setting of drowning merits study.
-
(3)
Is post-resuscitation therapeutic hypothermia effective in drowning? Studies in adults have reported efficacy after VF CA. Efficacy for other rhythms is more controversial. ACA data are limited. Benefit has been shown in perinatal asphyxia. However, there are differences between ACA in children and adults and birth asphyxia, which often represents a series of intermittent insults over several hours. It is unclear whether therapeutic hypothermia should be employed before RCTs have been conducted in ACA. Can mechanisms of hypothermic neuroprotection be better understood and efficacy improved [255–257]? Better definition of neuroprotective adjuncts to hypothermia is needed [228, 258]. If hypothermia is not used, should rigorous targeted normothermia be implemented [259]? For cold-water drowning, how should existing hypothermia and re-warming be managed [260]?
-
(4)
What is the best method to re-oxygenate the brain in drowning? VF studies indicate resuscitation with room air may be preferable to 100 % oxygen [261, 262]. ACA and drowning are different from VF with regard to respiratory physiology at the time of resuscitation. In ACA, the no-flow period is preceded by a period of anoxia. Pulmonary compromise may be present in drowning. AHA and ILCOR recommend titrated use of oxygen during VF resuscitation based on arterial saturation when pulse oximetry is available. This needs to be studied in drowning. There also may be developmental differences in antioxidant defenses. Might use of 100 % oxygen plus antioxidants be a better approach to oxygen administration?
-
(5)
How should reperfusion be optimized in drowning resuscitation? An opportunity for a breakthrough in resuscitation is use of extracorporeal support (E-CPR) for reperfusion, given that it can be used to optimize extra-cerebral physiology while also allowing immediate temperature control and administration of therapies that might not otherwise be tolerated [263–266]. Technical challenges to E-CPR are considerable. When is E-CPR indicated? Should “controlled reperfusion,” so as to limit reperfusion injury, be a research priority [267]? Some have suggested that reperfusion be totally re-thought. Should novel approaches, such as intermittent reperfusion (ischemic post-conditioning), be explored [268]?
-
(6)
What drugs might facilitate neurological recovery after ACA [233, 269]? Possibilities include laboratory and clinical investigation of drugs that are already approved for other uses that might be quickly brought to clinical trials, evaluation of novel therapies in laboratory ACA models, and investigation of the optimal rehabilitation of brain injury after drowning. Given neurogenesis and/or re-generative approaches to brain injury, optimizing the interaction between neuroprotection and neurorehabilitation deserves attention [269].
-
(7)
What novel resuscitation concepts should receive the highest priorities for exploration? Is it worthwhile to consider approaches that are “outside of the box”? Should novel non-pharmacological strategies such as the use of remote limb ischemia, so as to allow ischemic post-conditioning, be examined [270]? What novel pharmacological therapies under development for other forms of acute brain injury should be explored [271, 272]?
-
(8)
What is the best preclinical model for drowning research? Valid ACA models are available. Are there interactions between submersion, aspiration, and asphyxial arrest that are unique to neurologic outcome from drowning?
Evidence Basis for Consensus Recommendations
Experts in respective topics conducted focused literature reviews during a span of September 2011–February 2012 (search refreshed July 13, 2012). Databases searched included PubMed, Cochrane Library, and clinical trials.gov. Searches were limited to research on humans. Search terms incorporated key words associated with the topic (e.g., PEEP, hypoxia, hypothermia, etc.) combined with drown, drowning, near-drowning, asphyxia, HIE, or CA. To further refine the search, the terms brain or cerebral were included.
The search results were graded using the levels of evidence system reported by Sayre et al. [273] and modified as recommended for prognostic (P) applications, as appropriate.
-
LOE1: RCT or meta-analyses of RCTs.
-
LOE 2: Studies using concurrent controls without true randomization (e.g., “pseudo”-randomized).
-
LOE 3: Studies using retrospective controls.
-
LOE4: Studies without a control group (e.g., case series).
-
LOE 5: Studies not directly related to the specific patient population (e.g., different patient/population, animals models, mechanical models, etc.).
-
Expert Opinion
Each retrieved article was further graded with respect to whether the intervention was reported to provide beneficial, neutral, or harmful effects, or if effects were not specified [273]. Many of the studies rated as LOE 5, were judged as such because they were conducted in populations other than drowning. Often, such studies met the criteria for LOE 1 or LOE 2 in their respective populations. One goal of this search was to examine data directly derived from drowning and to demonstrate the general lack of information specific to this population. The reader is encouraged to carefully consider LOE 5 studies, as did members of the consensus committee in deriving recommendations, as such studies may provide the best evidence available to define treatment in drowning until information specific to drowning becomes available.
Prehospital Management
Prognostication
-
1 LOE 3 study in drowning [19].
Bystander CPR
-
1 LOE 4 study showing benefit in drowning [274].
Rescue Breathing During Bystander CPR
-
1 LOE 4 study showing benefit in drowning [20].
-
1 LOE 5 study showing benefit from compression only bystander CPR [21].
BiPaP or CPAP
Helicopter Transport
-
1 LOE 5 study showing benefit in CA [38].
Brain-Oriented Intensive Care Management
Positive End-Expiratory Pressure
Hypoxia or Hyperoxia
Arterial Hypotension
-
No studies in drowning.
-
2 LOE 5 studies showing neutral effects [279, 280], and 5 showing harm [32, 281–284] in CA.
Anti-epileptics
Strict Glucose Control
-
No studies in drowning.
-
1 LOE 5 study in CA showing neutral effects [295].
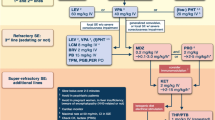
Therapeutic Hypothermia
-
2 LOE 3 studies showing neutral effects [85, 296], 1 LOE 4 report indicating benefit [297], 1 LOE 4 report indicating a neutral effect [298], and 7 LOE 4 studies for which the effect was not specified [184, 266, 299–303]. These studies were all in drowning.
-
25 LOE 5 studies showing benefit [87, 88, 90–96, 104, 226, 304–315], six LOE 5 studies showing neutral effect [89, 97–100, 103], 1 LOE 5 study showing harm [101], and 5 LOE 5 studies in which the effect was not indicted [147, 316–319]. These studies were in CA or birth asphyxia.
Neuromonitoring
-
1 LOE P2 study indicating benefit from neuromonitoring in drowning [154].
-
7 LOE P3 studies indicating benefit [120, 122, 123, 128, 132, 153, 320], 1 indicating neutral effects [121], and 1 indicating harm [85] in drowning.
-
5 LOE P4 studies indicating benefit [69, 118, 119, 144, 321]and one indicating a neutral effect [162] in drowning.
-
27 LOE P5 studies [70, 74, 75, 124, 125, 129–131, 136, 137, 139, 141, 142, 145–151, 322–328] and 1 expert opinion [71] indicating benefit, 4 LOE P5 indicating a neutral effects[68, 134, 143, 329], 1 LOE P5 indicating harm [152], and 1 LOE P5 not specified [140] in CA or HIE.
Post-Hypoxic Pharmacology
Antioxidants (Allopurinol, Ascorbate, Edaravone, Alpha-Tocopherol)
-
No studies have been conducted in drowning.
-
2 LOE 5 studies showing benefit [205, 207], and 3 showing a neutral effect in CA or HIE [203, 206, 208].
Barbiturates (Thiopental or Pentobarbital)
-
1 LOE 2 study showing neutral effects in drowning [184].
-
2 LOE 3 studies showing benefit [183, 330], and 1 showing a neutral effect [85] in drowning.
-
3 LOE 5 studies showing neutral effects in CA or HIE [184, 185, 187].
Calcium Channel Blockers (Nimodipine, Lidoflazine, Flunarizine)
-
1 LOE 4 study in drowning. The effect was not indicated [199].
-
2 LOE 5 studies showing benefit [185, 201], 1 showing harm [200] in CA or HIE.
Erythropoietin
-
No studies in drowning.
-
1 LOE 5 study showing benefit [217], and 3 showing a neutral effect in CA or HIE [214–216].
Glucocorticoids (Dexamethasone or Methylprednisolone)
-
1 LOE 4 study showing benefit in drowning [210].
-
3 LOE 5 studies showing neutral effects in CA or HIE [211, 212, 331].
Lidocaine
-
No studies in drowning, CA, or HIE.
Magnesium
References
Astrup J, Rehncrona S, Siesjo BK. The increase in extracellular potassium concentration in the ischemic brain in relation to the preischemic functional activity and cerebral metabolic rate. Brain Res. 1980;199(1):161–74.
Smith ML, Auer RN, Siesjo BK. The density and distribution of ischemic brain injury in the rat following 2–10 min of forebrain ischemia. Acta Neuropathol. 1984;64(4):319–32.
Petito CK, Feldmann E, Pulsinelli WA, Plum F. Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology. 1987;37(8):1281–6.
van Beeck EF, Branche CM, Szpilman D, Modell JH, Bierens JJ. A new definition of drowning: towards documentation and prevention of a global public health problem. Bull World Health Organ. 2005;83(11):853–6.
Papa L, Hoelle R, Idris A. Systematic review of definitions for drowning incidents. Resuscitation. 2005;65(3):255–64.
Idris AH, Berg RA, Bierens J, Bossaert L, Branche CM, Gabrielli A, et al. Recommended guidelines for uniform reporting of data from drowning: the “Utstein style”. Resuscitation. 2003;59(1):45–57.
Peden M, Oyegbite K, Ozanne-Smith J, Hyder AA, Branche CM, Rahman AKMF. World report on child injury prevention. Geneva: World Health Organization; 2008.
Hu Y, Wu L, Yu X, Zhang D, Liu X, Wang Y. Analysis of injury death trends among women in Macheng City, China, 1984–2008. BMC Public Health. 2011;11:698.
Liu Q, Zhang L, Li J, Zuo D, Kong D, Shen X, et al. The gap in injury mortality rates between urban and rural residents of Hubei Province, China. BMC Public Health. 2012;12:180.
Wang H, Smith GA, Stallones L, Xiang H. Injury-related childhood mortality in migrant households in a southern city of China. Inj Prev. 2010;16(3):161–5.
Jagnoor J, Suraweera W, Keay L, Ivers RQ, Thakur J, Jha P. Unintentional injury mortality in India, 2005: nationally representative mortality survey of 1.1 million homes. BMC Public Health. 2012;12(1):487.
Moller H, Larsen B, Helweg-Larsen K, Drowning in Denmark 2001–2009. Statens Institut for Folkesundhed, Syddansk Universitet (in Danish). http://www.worldconferenceondrowningprevention2011.org/SiteMedia/w3svc1092/Uploads/Documents/WCDP2011_Drown_R_Bech_p104_Abstract.pdf.
Bierens JJ, van der Velde EA, van Berkel M, van Zanten JJ. Submersion in The Netherlands: prognostic indicators and results of resuscitation. Ann Emerg Med. 1990;19(12):1390–5.
Nasrullah M, Muazzam S. Drowning mortality in the United States, 1999–2006. J Community Health. 2011;36(1):69–75.
Drowning—United States, 2005–2009, MMWR Morbidity and Mortality Weekly Report 2012;61:344–47
Bierens JJLM, van der Velde EA, van Berkel M, van Zanten JJ. Characteristics of submerged patients admitted to an intensive care unit. In: Bierens JJLM, editor. Drowning in the Netherlands. Pathophysiology, epidemiology and clinical studies. PhD Thesis University Utrecht, The Netherlands; 1996. p. 101–18. ISBN 90-393-1294-X.
Lu TH, Lunetta P, Walker S. Quality of cause-of-death reporting using ICD-10 drowning codes: a descriptive study of 69 countries. BMC Med Res Methodol. 2010;10:30.
Passmore JW, Smith JO, Clapperton A. True burden of drowning: compiling data to meet the new definition. Int J Inj Contr Saf Promot. 2007;14(1):1–3.
Quan L, Wentz KR, Gore EJ, Copass MK. Outcome and predictors of outcome in pediatric submersion victims receiving prehospital care in King County, Washington. Pediatrics. 1990;86(4):586–93.
March NF, Matthews RC. Feasibility study of CPR in the water. Undersea Biomed Res. 1980;7(2):141–8.
Hupfl M, Selig HF, Nagele P. Chest-compression-only versus standard cardiopulmonary resuscitation: a meta-analysis. Lancet. 2010;376(9752):1552–7.
Layon AJ, Modell JH. Drowning: update. Anesthesiology. 2009;110(6):1390–401.
Modell JH. Biology of drowning. Annu Rev Med. 1978;29:1–8.
Kitamura T, Iwami T, Kawamura T, Nagao K, Tanaka H, Hiraide A. Bystander-initiated rescue breathing for out-of-hospital cardiac arrests of noncardiac origin. Circulation. 2010;122(3):293–9.
Kitamura T, Iwami T, Kawamura T, Nagao K, Tanaka H, Nadkarni VM, et al. Conventional and chest-compression-only cardiopulmonary resuscitation by bystanders for children who have out-of-hospital cardiac arrests: a prospective, nationwide, population-based cohort study. Lancet. 2010;375(9723):1347–54.
Venema AM, Groothoff JW, Bierens JJ. The role of bystanders during rescue and resuscitation of drowning victims. Resuscitation. 2010;81(4):434–9.
Dottorini M, Eslami A, Baglioni S, Fiorenzano G, Todisco T. Nasal-continuous positive airway pressure in the treatment of near-drowning in freshwater. Chest. 1996;110(4):1122–4.
Poponick JM, Renston JP, Bennett RP, Emerman CL. Use of a ventilatory support system (BiPAP) for acute respiratory failure in the emergency department. Chest. 1999;116(1):166–71.
Baker PA, Webber JB. Failure to ventilate with supraglottic airways after drowning. Anaesth Intensive Care. 2011;39(4):675–7.
Kleinman ME, Chameides L, Schexnayder SM, Samson RA, Hazinski MF, Atkins DL, et al. Part 14: pediatric advanced life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S876–908.
Montenij LJ, de Vries W, Schwarte L, Bierens JJ. Feasibility of pulse oximetry in the initial prehospital management of victims of drowning: a preliminary study. Resuscitation. 2011;82(9):1235–8.
Trzeciak S, Jones AE, Kilgannon JH, Milcarek B, Hunter K, Shapiro NI, et al. Significance of arterial hypotension after resuscitation from cardiac arrest. Crit Care Med. 2009;37(11):2895–903. (quiz 2904).
Chochinov AH, Baydock BM, Bristow GK, Giesbrecht GG. Recovery of a 62-year-old man from prolonged cold water submersion. Ann Emerg Med. 1998;31(1):127–31.
Siebke H, Rod T, Breivik H, Link B. Survival after 40 minutes; submersion without cerebral sequeae. Lancet. 1975;1(7919):1275–7.
Gilbert M, Busund R, Skagseth A, Nilsen PA, Solbo JP. Resuscitation from accidental hypothermia of 13.7 degrees C with circulatory arrest [letter]. Lancet. 2000;355(9201):375–6.
Cabanas JG, Brice JH, De Maio VJ, Myers B, Hinchey PR. Field-induced therapeutic hypothermia for neuroprotection after out-of hospital cardiac arrest: a systematic review of the literature. J Emerg Med. 2011;40(4):400–9.
Wolff B, Machill K, Schumacher D, Schulzki I, Werner D. Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest. Int J Cardiol. 2009;133(2):223–8.
Werman HA, Falcone RA, Shaner S, Herron H, Johnson R, Lacey P, et al. Helicopter transport of patients to tertiary care centers after cardiac arrest. Am J Emerg Med. 1999;17(2):130–4.
Hasibeder WR. Drowning. Curr Opin Anaesthesiol. 2003;16(2):139–45.
Ender PT, Dolan MJ. Pneumonia associated with near-drowning. Clin Infect Dis. 1997;25(4):896–907.
Cortez KJ, Roilides E, Quiroz-Telles F, Meletiadis J, Antachopoulos C, Knudsen T, et al. Infections caused by Scedosporium spp. Clin Microbiol Rev. 2008;21(1):157–97.
Tadie JM, Heming N, Serve E, Weiss N, Day N, Imbert A, et al. Drowning associated pneumonia: a descriptive cohort. Resuscitation. 2012;83(3):399–401.
Suzuki H, Ohta T, Iwata K, Yamaguchi K, Sato T. Surfactant therapy for respiratory failure due to near-drowning. Eur J Pediatr. 1996;155(5):383–4.
Onarheim H, Vik V. Porcine surfactant (Curosurf) for acute respiratory failure after near-drowning in 12 year old. Acta Anaesthesiol Scand. 2004;48(6):778–81.
Varisco BM, Palmatier CM, Alten JA. Reversal of intractable hypoxemia with exogenous surfactant (calfactant) facilitating complete neurological recovery in a pediatric drowning victim. Pediatr Emerg Care. 2010;26(8):571–3.
Kesecioglu J, Beale R, Stewart TE, Findlay GP, Rouby JJ, Holzapfel L, et al. Exogenous natural surfactant for treatment of acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2009;180(10):989–94.
Spragg RG, Taut FJ, Lewis JF, Schenk P, Ruppert C, Dean N, et al. Recombinant surfactant protein C-based surfactant for patients with severe direct lung injury. Am J Respir Crit Care Med. 2011;183(8):1055–61.
Staudinger T, Bankier A, Strohmaier W, Weiss K, Locker GJ, Knapp S, et al. Exogenous surfactant therapy in a patient with adult respiratory distress syndrome after near drowning. Resuscitation. 1997;35(2):179–82.
Oba Y, Salzman GA. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–8.
Muench E, Bauhuf C, Roth H, Horn P, Phillips M, Marquetant N, et al. Effects of positive end-expiratory pressure on regional cerebral blood flow, intracranial pressure, and brain tissue oxygenation. Crit Care Med. 2005;33(10):2367–72.
Georgiadis D, Schwarz S, Baumgartner RW, Veltkamp R, Schwab S. Influence of positive end-expiratory pressure on intracranial pressure and cerebral perfusion pressure in patients with acute stroke. Stroke. 2001;32(9):2088–92.
Zhang XY, Yang ZJ, Wang QX, Fan HR. Impact of positive end-expiratory pressure on cerebral injury patients with hypoxemia. Am J Emerg Med. 2011;29(7):699–703.
Andrews PJ. Pressure, flow and Occam’s Razor: a matter of “steal”? Intensive Care Med. 2005;31(3):323–4.
Mascia L, Grasso S, Fiore T, Bruno F, Berardino M, Ducati A. Cerebro-pulmonary interactions during the application of low levels of positive end-expiratory pressure. Intensive Care Med. 2005;31(3):373–9.
Kilgannon JH, Jones AE, Parrillo JE, Dellinger RP, Milcarek B, Hunter K, et al. Relationship between supranormal oxygen tension and outcome after resuscitation from cardiac arrest. Circulation. 2011;123(23):2717–22.
Bisschops LL, Hoedemaekers CW, Simons KS, van der Hoeven JG. Preserved metabolic coupling and cerebrovascular reactivity during mild hypothermia after cardiac arrest. Crit Care Med. 2010;38(7):1542–7.
Bellomo R, Bailey M, Eastwood GM, Nichol A, Pilcher D, Hart GK, et al. Arterial hyperoxia and in-hospital mortality after resuscitation from cardiac arrest. Crit Care. 2011;15(2):R90.
Hoedemaekers CW, van der Hoeven JG. Hyperoxia after cardiac arrest may not increase ischemia-reperfusion injury. Crit Care. 2011;15(3):166.
Deakin CD, Nolan JP, Soar J, Sunde K, Koster RW, Smith GB, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 4. Adult advanced life support. Resuscitation. 2010;81(10):1305–52.
Peberdy MA, Callaway CW, Neumar RW, Geocadin RG, Zimmerman JL, Donnino M, et al. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S768–86.
Reinprecht A, Greher M, Wolfsberger S, Dietrich W, Illievich UM, Gruber A. Prone position in subarachnoid hemorrhage patients with acute respiratory distress syndrome: effects on cerebral tissue oxygenation and intracranial pressure. Crit Care Med. 2003;31(6):1831–8.
Fandel I, Bancalari E. Near-drowning in children: clinical aspects. Pediatrics. 1976;58(4):573–9.
Tabeling BB, Modell JH. Fluid administration increases oxygen delivery during continuous positive pressure ventilation after freshwater near-drowning. Crit Care Med. 1983;11(9):693–6.
Nussbaum E. Prognostic variables in nearly drowned, comatose children. Am J Dis Child. 1985;139(10):1058–9.
Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–75.
Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62(10):1743–8.
Jette N, Claassen J, Emerson RG, Hirsch LJ. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol. 2006;63(12):1750–5.
Abend NS, Topjian A, Ichord R, Herman ST, Helfaer M, Donnelly M, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology. 2009;72(22):1931–40.
Janati A, Erba G. Electroencephalographic correlates of near-drowning encephalopathy in children. Electroencephalogr Clin Neurophysiol. 1982;53(2):182–91.
Zandbergen EG, Hijdra A, Koelman JH, Hart AA, Vos PE, Verbeek MM, et al. Prediction of poor outcome within the first 3 days of postanoxic coma. Neurology. 2006;66(1):62–8.
Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67(2):203–10.
Wijdicks EF, Young GB. Myoclonus status in comatose patients after cardiac arrest. Lancet. 1994;343(8913):1642–3.
Rossetti AO, Oddo M, Liaudet L, Kaplan PW. Predictors of awakening from postanoxic status epilepticus after therapeutic hypothermia. Neurology. 2009;72(8):744–9.
Rossetti AO, Urbano LA, Delodder F, Kaplan PW, Oddo M. Prognostic value of continuous EEG monitoring during therapeutic hypothermia after cardiac arrest. Crit Care. 2010;14(5):R173.
Rundgren M, Westhall E, Cronberg T, Rosen I, Friberg H. Continuous amplitude-integrated electroencephalogram predicts outcome in hypothermia-treated cardiac arrest patients. Crit Care Med. 2010;38(9):1838–44.
Saidel-Odes LR, Almog Y. Near-drowning in the Dead Sea: a retrospective observational analysis of 69 patients. Isr Med Assoc J. 2003;5(12):856–8.
Thiele RH, Pouratian N, Zuo Z, Scalzo DC, Dobbs HA, Dumont AS, et al. Strict glucose control does not affect mortality after aneurysmal subarachnoid hemorrhage. Anesthesiology. 2009;110(3):603–10.
Bilotta F, Caramia R, Paoloni FP, Delfini R, Rosa G. Safety and efficacy of intensive insulin therapy in critical neurosurgical patients. Anesthesiology. 2009;110(3):611–9.
Meierhans R, Bechir M, Ludwig S, Sommerfeld J, Brandi G, Haberthur C, et al. Brain metabolism is significantly impaired at blood glucose below 6 mM and brain glucose below 1 mM in patients with severe traumatic brain injury. Crit Care. 2010;14(1):R13.
Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012;13(4):267–78.
Holzer M. Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med. 2010;363(13):1256–64.
Rosomoff HL, Safar P. Management of the comatose patient. Clin Anesth. 1965;1:244–58.
Safar P. Community-wide cardiopulmonary resuscitation. J Iowa Med Soc. 1964;54:629–35.
Conn AW, Edmonds JF, Barker GA. Cerebral resuscitation in near-drowning. Pediatr Clin North Am. 1979;26(3):691–701.
Bohn DJ, Biggar WD, Smith CR, Conn AW, Barker GA. Influence of hypothermia, barbiturate therapy, and intracranial pressure monitoring on morbidity and mortality after near-drowning. Crit Care Med. 1986;14(6):529–34.
Biggart MJ, Bohn DJ. Effect of hypothermia and cardiac arrest on outcome of near-drowning accidents in children. J Pediatr. 1990;117(2 Pt 1):179–83.
Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–56.
Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–63.
Doherty DR, Parshuram CS, Gaboury I, Hoskote A, Lacroix J, Tucci M, et al. Hypothermia therapy after pediatric cardiac arrest. Circulation. 2009;119(11):1492–500.
Jacobs S, Hunt R, Tarnow-Mordi W, Inder T, Davis P. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2007;4:CD003311.
Shah PS. Hypothermia: a systematic review and meta-analysis of clinical trials. Semin Fetal Neonatal Med. 2010;15(5):238–46.
Gunn AJ, Gluckman PD, Gunn TR. Selective head cooling in newborn infants after perinatal asphyxia: a safety study. Pediatrics. 1998;102(4 Pt 1):885–92.
Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic–ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–84.
Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–70.
Lin ZL, Yu HM, Lin J, Chen SQ, Liang ZQ, Zhang ZY. Mild hypothermia via selective head cooling as neuroprotective therapy in term neonates with perinatal asphyxia: an experience from a single neonatal intensive care unit. J Perinatol. 2006;26(3):180–4.
Zhou WH, Cheng GQ, Shao XM, Liu XZ, Shan RB, Zhuang DY et al., Selective head cooling with mild systemic hypothermia after neonatal hypoxic–ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr 2010;157(3):367–372, 372.e1–3
Eicher DJ, Wagner CL, Katikaneni LP, Hulsey TC, Bass WT, Kaufman DA, et al. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005;32(1):11–7.
Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349–58.
Battin MR, Dezoete JA, Gunn TR, Gluckman PD, Gunn AJ. Neurodevelopmental outcome of infants treated with head cooling and mild hypothermia after perinatal asphyxia. Pediatrics. 2001;107(3):480–4.
Battin MR, Penrice J, Gunn TR, Gunn AJ. Treatment of term infants with head cooling and mild systemic hypothermia (35.0 degrees C and 34.5 degrees C) after perinatal asphyxia. Pediatrics. 2003;111(2):244–51.
Robertson NJ, Nakakeeto M, Hagmann C, Cowan FM, Acolet D, Iwata O, et al. Therapeutic hypothermia for birth asphyxia in low-resource settings: a pilot randomised controlled trial. Lancet. 2008;372(9641):801–3.
Robertson NJ, Hagmann CF, Acolet D, Allen E, Nyombi N, Elbourne D, et al. Pilot randomized trial of therapeutic hypothermia with serial cranial ultrasound and 18–22 month follow-up for neonatal encephalopathy in a low resource hospital setting in Uganda: study protocol. Trials. 2011;12:138.
Hachimi-Idrissi S, Corne L, Ebinger G, Michotte Y, Huyghens L. Mild hypothermia induced by a helmet device: a clinical feasibility study. Resuscitation. 2001;51(3):275–81.
Arrich J, Holzer M, Herkner H, Mullner M. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2009;4:CD004128.
ERC Guidelines Writing Group. European Resuscitation Council Guidelines for Resuscitation 2010. Resuscitation. 2010;81:1219–451.
Nielsen N, Friberg H, Gluud C, Herlitz J, Wetterslev J. Hypothermia after cardiac arrest should be further evaluated—a systematic review of randomised trials with meta-analysis and trial sequential analysis. Int J Cardiol. 2011;151(3):333–41.
Weiss J. Prevention of drowning. Pediatrics. 2010;126(1):e253–62.
Bowman SM, Aitken ME, Robbins JM, Baker SP. Trends in US pediatric drowning hospitalizations, 1993–2008. Pediatrics. 2012;129(2):275–81.
Present P. Child drowning study: a report on the epidemiology of drownings in residential pools to children under age five. Washington, DC: US Consumer Product Safety Commission; 1987.
Quan L, Gore EJ, Wentz K, Allen J, Novack AH. Ten-year study of pediatric drownings and near-drownings in King County, Washington: lessons in injury prevention. Pediatrics. 1989;83(6):1035–40.
Moler FW, Donaldson AE, Meert K, Brilli RJ, Nadkarni V, Shaffner DH, et al. Multicenter cohort study of out-of-hospital pediatric cardiac arrest. Crit Care Med. 2011;39(1):141–9.
Gooden BA. Why some people do not drown. Hypothermia versus the diving response. Med J Aust. 1992;157(9):629–32.
Berger RP, Bazaco MC, Wagner AK, Kochanek PM, Fabio A. Trajectory analysis of serum biomarker concentrations facilitates outcome prediction after pediatric traumatic and hypoxemic brain injury. Dev Neurosci. 2010;32(5–6):396–405.
Topjian AA, Lin R, Morris MC, Ichord R, Drott H, Bayer CR, et al. Neuron-specific enolase and S-100B are associated with neurologic outcome after pediatric cardiac arrest. Pediatr Crit Care Med. 2009;10(4):479–90.
Peters A, Schweiger U, Fruhwald-Schultes B, Born J, Fehm HL. The neuroendocrine control of glucose allocation. Exp Clin Endocrinol Diabetes. 2002;110(5):199–211.
White H, Venkatesh B. Clinical review: ketones and brain injury. Crit Care. 2011;15(2):219.
van Velthoven CT, Kavelaars A, Heijnen CJ. Mesenchymal stem cells as a treatment for neonatal ischemic brain damage. Pediatr Res. 2012;71(4 Pt 2):474–81.
Peterson B. Morbidity of childhood near-drowning. Pediatrics. 1977;59(3):364–70.
Modell JH, Graves SA, Ketover A. Clinical course of 91 consecutive near-drowning victims. Chest. 1976;70(2):231–8.
Graf WD, Cummings P, Quan L, Brutocao D. Predicting outcome in pediatric submersion victims. Ann Emerg Med. 1995;26(3):312–9.
Frates RC Jr. Analysis of predictive factors in the assessment of warm-water near-drowning in children. Am J Dis Child. 1981;135(11):1006–8.
Lavelle JM, Shaw KN. Near drowning: is emergency department cardiopulmonary resuscitation or intensive care unit cerebral resuscitation indicated? Crit Care Med. 1993;21(3):368–73.
Ballesteros MA, Gutierrez-Cuadra M, Munoz P, Minambres E. Prognostic factors and outcome after drowning in an adult population. Acta Anaesthesiol Scand. 2009;53(7):935–40.
Edgren E, Hedstrand U, Kelsey S, Sutton-Tyrrell K, Safar P. Assessment of neurological prognosis in comatose survivors of cardiac arrest. BRCT I Study Group. Lancet. 1994;343(8905):1055–9.
Mandel R, Martinot A, Delepoulle F, Lamblin MD, Laureau E, Vallee L, et al. Prediction of outcome after hypoxic–ischemic encephalopathy: a prospective clinical and electrophysiologic study. J Pediatr. 2002;141(1):45–50.
Rossetti AO, Oddo M, Logroscino G, Kaplan PW. Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol. 2010;67(3):301–7.
Christensen DW, Jansen P, Perkin RM. Outcome and acute care hospital costs after warm water near drowning in children. Pediatrics. 1997;99(5):715–21.
Bratton SL, Jardine DS, Morray JP. Serial neurologic examinations after near drowning and outcome. Arch Pediatr Adolesc Med. 1994;148(2):167–70.
Levy DE, Caronna JJ, Singer BH, Lapinski RH, Frydman H, Plum F. Predicting outcome from hypoxic–ischemic coma. JAMA. 1985;253(10):1420–6.
Bassetti C, Bomio F, Mathis J, Hess CW. Early prognosis in coma after cardiac arrest: a prospective clinical, electrophysiological, and biochemical study of 60 patients. J Neurol Neurosurg Psychiatry. 1996;61(6):610–5.
Toet MC, Hellstrom-Westas L, Groenendaal F, Eken P, de Vries LS. Amplitude integrated EEG 3 and 6 hours after birth in full term neonates with hypoxic–ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 1999;81(1):F19–23.
Cheliout-Heraut F, Sale-Franque F, Hubert P, Bataille J. Cerebral anoxia in near-drowning of children. The prognostic value of EEG. Neurophysiol Clin. 1991;21(2):121–32.
Young GB, Doig G, Ragazzoni A. Anoxic–ischemic encephalopathy: clinical and electrophysiological associations with outcome. Neurocrit Care. 2005;2(2):159–64.
Rothstein TL, Thomas EM, Sumi SM. Predicting outcome in hypoxic–ischemic coma. A prospective clinical and electrophysiologic study. Electroencephalogr Clin Neurophysiol. 1991;79(2):101–7.
Nishisaki A, Sullivan J III, Steger B, Bayer CR, Dlugos D, Lin R, et al. Retrospective analysis of the prognostic value of electroencephalography patterns obtained in pediatric in-hospital cardiac arrest survivors during three years. Pediatr Crit Care Med. 2007;8(1):10–7.
Kessler SK, Topjian AA, Gutierrez-Colina AM, Ichord RN, Donnelly M, Nadkarni VM, et al. Short-term outcome prediction by electroencephalographic features in children treated with therapeutic hypothermia after cardiac arrest. Neurocrit Care. 2011;14(1):37–43.
Thenayan EA, Savard M, Sharpe MD, Norton L, Young B. Electroencephalogram for prognosis after cardiac arrest. J Crit Care. 2010;25(2):300–4.
Taylor SB, Quencer RM, Holzman BH, Naidich TP. Central nervous system anoxic–ischemic insult in children due to near-drowning. Radiology. 1985;156(3):641–6.
Rafaat KT, Spear RM, Kuelbs C, Parsapour K, Peterson B. Cranial computed tomographic findings in a large group of children with drowning: diagnostic, prognostic, and forensic implications. Pediatr Crit Care Med. 2008;9(6):567–72.
Cocchi MN, Lucas JM, Salciccioli J, Carney E, Herman S, Zimetbaum P, et al. The role of cranial computed tomography in the immediate post-cardiac arrest period. Int Emerg Med. 2010;5(6):533–8.
Choi SP, Park HK, Park KN, Kim YM, Ahn KJ, Choi KH, et al. The density ratio of grey to white matter on computed tomography as an early predictor of vegetative state or death after cardiac arrest. Emerg Med J. 2008;25(10):666–9.
Fugate JE, Wijdicks EF, Mandrekar J, Claassen DO, Manno EM, White RD, et al. Predictors of neurologic outcome in hypothermia after cardiac arrest. Ann Neurol. 2010;68(6):907–14.
Inamasu J, Miyatake S, Suzuki M, Nakatsukasa M, Tomioka H, Honda M, et al. Early CT signs in out-of-hospital cardiac arrest survivors: temporal profile and prognostic significance. Resuscitation. 2010;81(5):534–8.
Fitch SJ, Gerald B, Magill HL, Tonkin IL. Central nervous system hypoxia in children due to near drowning. Radiology. 1985;156(3):647–50.
Han BK, Towbin RB, De Courten-Myers G, McLaurin RL, Ball WS Jr. Reversal sign on CT: effect of anoxic/ischemic cerebral injury in children. AJR Am J Roentgenol. 1990;154(2):361–8.
Metter RB, Rittenberger JC, Guyette FX, Callaway CW. Association between a quantitative CT scan measure of brain edema and outcome after cardiac arrest. Resuscitation. 2011;82(9):1180–5.
Baldursdottir S, Sigvaldason K, Karason S, Valsson F, Sigurdsson GH. Induced hypothermia in comatose survivors of asphyxia: a case series of 14 consecutive cases. Acta Anaesthesiol Scand. 2010;54(7):821–6.
Wijman CA, Mlynash M, Caulfield AF, Hsia AW, Eyngorn I, Bammer R, et al. Prognostic value of brain diffusion-weighted imaging after cardiac arrest. Ann Neurol. 2009;65(4):394–402.
Wu O, Sorensen AG, Benner T, Singhal AB, Furie KL, Greer DM. Comatose patients with cardiac arrest: predicting clinical outcome with diffusion-weighted MR imaging. Radiology. 2009;252(1):173–81.
Choi SP, Park KN, Park HK, Kim JY, Youn CS, Ahn KJ, et al. Diffusion-weighted magnetic resonance imaging for predicting the clinical outcome of comatose survivors after cardiac arrest: a cohort study. Crit Care. 2010;14(1):R17.
Mlynash M, Campbell DM, Leproust EM, Fischbein NJ, Bammer R, Eyngorn I, et al. Temporal and spatial profile of brain diffusion-weighted MRI after cardiac arrest. Stroke. 2010;41(8):1665–72.
Christophe C, Fonteyne C, Ziereisen F, Christiaens F, Deltenre P, De Maertelaer V, et al. Value of MR imaging of the brain in children with hypoxic coma. AJNR Am J Neuroradiol. 2002;23(4):716–23.
Dubowitz DJ, Bluml S, Arcinue E, Dietrich RB. MR of hypoxic encephalopathy in children after near drowning: correlation with quantitative proton MR spectroscopy and clinical outcome. AJNR Am J Neuroradiol. 1998;19(9):1617–27.
Kreis R, Arcinue E, Ernst T, Shonk TK, Flores R, Ross BD. Hypoxic encephalopathy after near-drowning studied by quantitative 1H-magnetic resonance spectroscopy. J Clin Invest. 1996;97(5):1142–54.
Bouwes A, Binnekade JM, Verbaan BW, Zandbergen EG, Koelman JH, Weinstein HC, et al. Predictive value of neurological examination for early cortical responses to somatosensory evoked potentials in patients with postanoxic coma. J Neurol. 2012;259:537–41.
Carter BG, Butt W. A prospective study of outcome predictors after severe brain injury in children. Intensive Care Med. 2005;31(6):840–5.
Dean JM, McComb JG. Intracranial pressure monitoring in severe pediatric near-drowning. Neurosurgery. 1981;9(6):627–30.
Le Roux PD, Jardine DS, Kanev PM, Loeser JD. Pediatric intracranial pressure monitoring in hypoxic and nonhypoxic brain injury. Childs Nerv Syst. 1991;7(1):34–9.
Nussbaum E, Galant SP. Intracranial pressure monitoring as a guide to prognosis in the nearly drowned, severely comatose child. J Pediatr. 1983;102(2):215–8.
Allman FD, Nelson WB, Pacentine GA, McComb G. Outcome following cardiopulmonary resuscitation in severe pediatric near-drowning. Am J Dis Child. 1986;140(6):571–5.
Tasker RC, Matthew DJ, Helms P, Dinwiddie R, Boyd S. Monitoring in non-traumatic coma. Part I: invasive intracranial measurements. Arch Dis Child. 1988;63(8):888–94.
Sarnaik AP, Preston G, Lieh-Lai M, Eisenbrey AB. Intracranial pressure and cerebral perfusion pressure in near-drowning. Crit Care Med. 1985;13(4):224–7.
Durward QJ, Amacher AL, Del Maestro RF, Sibbald WJ. Cerebral and cardiovascular responses to changes in head elevation in patients with intracranial hypertension. J Neurosurg. 1983;59(6):938–44.
Mizuta R, Fujita H, Osamura T, Kidowaki T, Kiyosawa N. Childhood drownings and near-drownings in Japan. Acta Paediatr Jpn. 1993;35(3):186–92.
Checchia PA, Moynihan JA, Brown L. Cardiac troponin I as a predictor of mortality for pediatric submersion injuries requiring out-of-hospital cardiopulmonary resuscitation. Pediatr Emerg Care. 2006;22(4):222–5.
Rundgren M, Karlsson T, Nielsen N, Cronberg T, Johnsson P, Friberg H. Neuron specific enolase and S-100B as predictors of outcome after cardiac arrest and induced hypothermia. Resuscitation. 2009;80(7):784–9.
Morrison LJ, Deakin CD, Morley PT, Callaway CW, Kerber RE, Kronick SL, et al. Part 8: advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010;122(16 Suppl 2):S345–421.
Oksanen T, Tiainen M, Skrifvars MB, Varpula T, Kuitunen A, Castren M, et al. Predictive power of serum NSE and OHCA score regarding 6-month neurologic outcome after out-of-hospital ventricular fibrillation and therapeutic hypothermia. Resuscitation. 2009;80(2):165–70.
Daubin C, Quentin C, Allouche S, Etard O, Gaillard C, Seguin A, et al. Serum neuron-specific enolase as predictor of outcome in comatose cardiac-arrest survivors: a prospective cohort study. BMC Cardiovasc Disord. 2011;11:48.
Steffen IG, Hasper D, Ploner CJ, Schefold JC, Dietz E, Martens F, et al. Mild therapeutic hypothermia alters neuron specific enolase as an outcome predictor after resuscitation: 97 prospective hypothermia patients compared to 133 historical non-hypothermia patients. Crit Care. 2010;14(2):R69.
Cronberg T, Rundgren M, Westhall E, Englund E, Siemund R, Rosen I, et al. Neuron-specific enolase correlates with other prognostic markers after cardiac arrest. Neurology. 2011;77(7):623–30.
Friberg H, Rundgren M. Submersion, accidental hypothermia and cardiac arrest, mechanical chest compressions as a bridge to final treatment: a case report. Scand J Trauma Resusc Emerg Med. 2009;17:7.
Shinozaki K, Oda S, Sadahiro T, Nakamura M, Abe R, Nakada TA, et al. Serum S-100B is superior to neuron-specific enolase as an early prognostic biomarker for neurological outcome following cardiopulmonary resuscitation. Resuscitation. 2009;80(8):870–5.
Tiainen M, Roine RO, Pettila V, Takkunen O. Serum neuron-specific enolase and S-100B protein in cardiac arrest patients treated with hypothermia. Stroke. 2003;34(12):2881–6.
Kaneko T, Kasaoka S, Miyauchi T, Fujita M, Oda Y, Tsuruta R, et al. Serum glial fibrillary acidic protein as a predictive biomarker of neurological outcome after cardiac arrest. Resuscitation. 2009;80(7):790–4.
Mörtberg E, Zetterberg H, Nordmark J, Blennow K, Catry C, Decraemer H, Vanmechelen E, Rubertsson S. Plasma tau protein in comatose patients after cardiac arrest treated with therapeutic hypothermia. Acta Anaesthesiol Scand. 2011;55(9):1132–8.
Ashwal S, Schneider S, Tomasi L, Thompson J. Prognostic implications of hyperglycemia and reduced cerebral blood flow in childhood near-drowning. Neurology. 1990;40(5):820–3.
Wollenek G, Honarwar N, Golej J, Marx M. Cold water submersion and cardiac arrest in treatment of severe hypothermia with cardiopulmonary bypass. Resuscitation. 2002;52(3):255–63.
Fries M, Kunz D, Gressner AM, Rossaint R, Kuhlen R. Procalcitonin serum levels after out-of-hospital cardiac arrest. Resuscitation. 2003;59(1):105–9.
Los Arcos M, Rey C, Concha A, Medina A, Prieto B. Acute-phase reactants after paediatric cardiac arrest. Procalcitonin as marker of immediate outcome. BMC Pediatr. 2008;8:18.
Gidlof O, Andersson P, van der Pals J, Gotberg M, Erlinge D. Cardiospecific microRNA plasma levels correlate with troponin and cardiac function in patients with st elevation myocardial infarction, are selectively dependent on renal elimination, and can be detected in urine samples. Cardiology. 2011;118(4):217–26.
Conn AW, Edmonds JF, Barker GA. Near-drowning in cold fresh water: current treatment regimen. Can Anaesth Soc J. 1978;25(4):259–65.
Conn AW, Montes JE, Barker GA, Edmonds JF. Cerebral salvage in near-drowning following neurological classification by triage. Can Anaesth Soc J. 1980;27(3):201–10.
Nussbaum E, Maggi JC. Pentobarbital therapy does not improve neurologic outcome in nearly drowned, flaccid-comatose children. Pediatrics. 1988;81(5):630–4.
Brain Resuscitation Clinical Trial I Study Group. Randomized clinical study of thiopental loading in comatose survivors of cardiac arrest. N Engl J Med. 1986;314(7):397–403.
Michenfelder JD. Positive experimental demonstration of the negative brain “protective” effects of anesthetics following cardiac arrest. Anesthesiology. 2002;97(4):1005–6.
Goldberg RN, Moscoso P, Bauer CR, Bloom FL, Curless RG, Burke B, et al. Use of barbiturate therapy in severe perinatal asphyxia: a randomized controlled trial. J Pediatr. 1986;109(5):851–6.
Ruth V, Virkola K, Paetau R, Raivio KO. Early high-dose phenobarbital treatment for prevention of hypoxic–ischemic brain damage in very low birth weight infants. J Pediatr. 1988;112(1):81–6.
Mitchell SJ, Pellett O, Gorman DF. Cerebral protection by lidocaine during cardiac operations. Ann Thorac Surg. 1999;67(4):1117–24.
Mathew JP, Mackensen GB, Phillips-Bute B, Grocott HP, Glower DD, Laskowitz DT, et al. Randomized, double-blinded, placebo controlled study of neuroprotection with lidocaine in cardiac surgery. Stroke. 2009;40(3):880–7.
Astrup J, Sorensen PM, Sorensen HR. Inhibition of cerebral oxygen and glucose consumption in the dog by hypothermia, pentobarbital, and lidocaine. Anesthesiology. 1981;55(3):263–8.
Groenendaal F, Rademaker CM, Toet MC, de Vries LS. Effects of magnesium sulphate on amplitude-integrated continuous EEG in asphyxiated term neonates. Acta Paediatr. 2002;91(10):1073–7.
Ichiba H, Tamai H, Negishi H, Ueda T, Kim TJ, Sumida Y, et al. Randomized controlled trial of magnesium sulfate infusion for severe birth asphyxia. Pediatr Int. 2002;44(5):505–9.
Bhat MA, Charoo BA, Bhat JI, Ahmad SM, Ali SW, Mufti MU. Magnesium sulfate in severe perinatal asphyxia: a randomized, placebo-controlled trial. Pediatrics. 2009;123(5):e764–9.
Gathwala G, Khera A, Singh J, Balhara B. Magnesium for neuroprotection in birth asphyxia. J Pediatr Neurosci. 2010;5(2):102–4.
Longstreth WT Jr, Fahrenbruch CE, Olsufka M, Walsh TR, Copass MK, Cobb LA. Randomized clinical trial of magnesium, diazepam, or both after out-of-hospital cardiac arrest. Neurology. 2002;59(4):506–14.
Temkin NR, Anderson GD, Winn HR, Ellenbogen RG, Britz GW, Schuster J, et al. Magnesium sulfate for neuroprotection after traumatic brain injury: a randomised controlled trial. Lancet Neurol. 2007;6(1):29–38.
Meloni BP, Campbell K, Zhu H, Knuckey NW. In search of clinical neuroprotection after brain ischemia: the case for mild hypothermia (35 degrees C) and magnesium. Stroke. 2009;40(6):2236–40.
Kollar DJ. Cerebral resuscitation by use of verapamil in a victim of near-drowning. Am J Emerg Med. 1984;2(2):148–52.
Levene MI, Gibson NA, Fenton AC, Papathoma E, Barnett D. The use of a calcium-channel blocker, nicardipine, for severely asphyxiated newborn infants. Dev Med Child Neurol. 1990;32(7):567–74.
Roine RO, Kaste M, Kinnunen A, Nikki P, Sarna S, Kajaste S. Nimodipine after resuscitation from out-of-hospital ventricular fibrillation. A placebo-controlled, double-blind, randomized trial. JAMA. 1990;264(24):3171–7.
Brain Resuscitation Clinical Trial II Study Group. A randomized clinical study of a calcium-entry blocker (lidoflazine) in the treatment of comatose survivors of cardiac arrest. N Engl J Med. 1991;324(18):1225–31.
Aly H, Abd-Rabboh L, El-Dib M, Nawwar F, Hassan H, Aaref M, et al. Ascorbic acid combined with ibuprofen in hypoxic ischemic encephalopathy: a randomized controlled trial. J Perinatol. 2009;29(6):438–43.
Warner DS, Sheng H, Batinic-Haberle I. Oxidants, antioxidants and the ischemic brain. J Exp Biol. 2004;207(Pt 18):3221–31.
van Bel F, Shadid M, Moison RM, Dorrepaal CA, Fontijn J, Monteiro L, et al. Effect of allopurinol on postasphyxial free radical formation, cerebral hemodynamics, and electrical brain activity. Pediatrics. 1998;101:185–93.
Benders MJ, Bos AF, Rademaker CM, Rijken M, Torrance HL, Groenendaal F, et al. Early postnatal allopurinol does not improve short term outcome after severe birth asphyxia. Arch Dis Child Fetal Neonatal Ed. 2006;91(3):F163–5.
Gunes T, Ozturk MA, Koklu E, Kose K, Gunes I. Effect of allopurinol supplementation on nitric oxide levels in asphyxiated newborns. Pediatr Neurol. 2007;36(1):17–24.
Chaudhari T, McGuire W. Allopurinol for preventing mortality and morbidity in newborn infants with suspected hypoxic–ischaemic encephalopathy. Cochrane Database Syst Rev. 2008;2:CD006817.
Kaandorp JJ, Benders MJ, Rademaker CM, Torrance HL, Oudijk MA, de Haan TR, et al. Antenatal allopurinol for reduction of birth asphyxia induced brain damage (ALLO-Trial); a randomized double blind placebo controlled multicenter study. BMC Pregnancy Childbirth. 2010;10:8.
Pfenninger J, Sutter M. Intensive care after fresh water immersion accidents in children. Anaesthesia. 1982;37(12):1157–62.
Grafton ST, Longstreth WT Jr. Steroids after cardiac arrest: a retrospective study with concurrent, nonrandomized controls. Neurology. 1988;38(8):1315–6.
Jastremski M, Sutton-Tyrrell K, Vaagenes P, Abramson N, Heiselman D, Safar P. Glucocorticoid treatment does not improve neurological recovery following cardiac arrest. Brain Resuscitation Clinical Trial I Study Group. JAMA. 1989;262(24):3427–30.
Xanthos T, Vasileiou PV, Kakavas S, Syggelou A, Iacovidou N. The potential role of erythropoietin as a pleiotropic agent in post-cardiac arrest syndrome. Curr Pharm Des. 2011;17(15):1517–29.
Grmec S, Strnad M, Kupnik D, Sinkovic A, Gazmuri RJ. Erythropoietin facilitates the return of spontaneous circulation and survival in victims of out-of-hospital cardiac arrest. Resuscitation. 2009;80(6):631–7.
Cariou A, Claessens YE, Pene F, Marx JS, Spaulding C, Hababou C, et al. Early high-dose erythropoietin therapy and hypothermia after out-of-hospital cardiac arrest: a matched control study. Resuscitation. 2008;76(3):397–404.
Zhu C, Kang W, Xu F, Cheng X, Zhang Z, Jia L, et al. Erythropoietin improved neurologic outcomes in newborns with hypoxic–ischemic encephalopathy. Pediatrics. 2009;124(2):e218–26.
Elmahdy H, El-Mashad AR, El-Bahrawy H, El-Gohary T, El-Barbary A, Aly H. Human recombinant erythropoietin in asphyxia neonatorum: pilot trial. Pediatrics. 2010;125(5):e1135–42.
Tisherman S, Chabal C, Safar P, Stezoski W. Resuscitation of dogs from cold-water submersion using cardiopulmonary bypass. Ann Emerg Med. 1985;14(5):389–96.
Berg RA, Hilwig RW, Kern KB, Ewy GA. “Bystander” chest compressions and assisted ventilation independently improve outcome from piglet asphyxial pulseless “cardiac arrest”. Circulation. 2000;101(14):1743–8.
Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest. 1997;99(2):248–56.
Young RS, Zalneraitis EL, Dooling EC. Neurological outcome in cold water drowning. JAMA. 1980;244(11):1233–5.
Hickey RW, Akino M, Strausbaugh S, De Courten-Myers GM. Use of the Morris water maze and acoustic startle chamber to evaluate neurologic injury after asphyxial arrest in rats. Pediatr Res. 1996;39(1):77–84.
Hickey RW, Ferimer H, Alexander HL, Garman RH, Callaway CW, Hicks S, et al. Delayed, spontaneous hypothermia reduces neuronal damage after asphyxial cardiac arrest in rats. Crit Care Med. 2000;28(10):3511–6.
Katz L, Ebmeyer U, Safar P, Radovsky A, Neumar R. Outcome model of asphyxial cardiac arrest in rats. J Cereb Blood Flow Metab. 1995;15(6):1032–9.
Katz L, Vaagenes P, Safar P, Diven W. Brain enzyme changes as markers of brain damage in rat cardiac arrest model. Effects of corticosteroid therapy. Resuscitation. 1989;17(1):39–53.
Xiao F, Safar P, Radovsky A. Mild protective and resuscitative hypothermia for asphyxial cardiac arrest in rats. Am J Emerg Med. 1998;16(1):17–25.
Katz LM, Wang Y, Ebmeyer U, Radovsky A, Safar P. Glucose plus insulin infusion improves cerebral outcome after asphyxial cardiac arrest. NeuroReport. 1998;9(15):3363–7.
Katz LM, Wang Y, McMahon B, Richelson E. Neurotensin analog NT69L induces rapid and prolonged hypothermia after hypoxic ischemia. Acad Emerg Med. 2001;8(12):1115–21.
Katz LM, Young A, Frank JE, Wang Y, Park K. Neurotensin-induced hypothermia improves neurologic outcome after hypoxic–ischemia. Crit Care Med. 2004;32(3):806–10.
Fink EL, Alexander H, Marco CD, Dixon CE, Kochanek PM, Jenkins LW, et al. Experimental model of pediatric asphyxial cardiopulmonary arrest in rats. Pediatr Crit Care Med. 2004;5(2):139–44.
Fink EL, Marco CD, Donovan HA, Alexander H, Dixon CE, Jenkins LW, et al. Brief induced hypothermia improves outcome after asphyxial cardiopulmonary arrest in juvenile rats. Dev Neurosci. 2005;27(2–4):191–9.
Manole MD, Foley LM, Hitchens TK, Kochanek PM, Hickey RW, Bayir H, et al. Magnetic resonance imaging assessment of regional cerebral blood flow after asphyxial cardiac arrest in immature rats. J Cereb Blood Flow Metab. 2009;29(1):197–205.
Tang M, Alexander H, Clark RS, Kochanek PM, Kagan VE, Bayir H. Minocycline reduces neuronal death and attenuates microglial response after pediatric asphyxial cardiac arrest. J Cereb Blood Flow Metab. 2010;30(1):119–29.
Albaghdadi AS, Brooks LA, Pretorius AM, Kerber RE. Perfluorocarbon induced intra-arrest hypothermia does not improve survival in a swine model of asphyxial cardiac arrest. Resuscitation. 2010;81(3):353–8.
Chen KT, Lin HJ, Jeng HW, Lin CC, Guo HR. The pharmacological effect of epinephrine administration via laryngeal mask airway in a porcine model of asphyxial cardiac arrest. Emerg Med J. 2008;25(11):722–4.
Mayr VD, Wenzel V, Voelckel WG, Krismer AC, Mueller T, Lurie KG, et al. Developing a vasopressor combination in a pig model of adult asphyxial cardiac arrest. Circulation. 2001;104(14):1651–6.
Mayr VD, Mitterschiffthaler L, Neurauter A, Gritsch C, Wenzel V, Muller T, et al. A comparison of the combination of epinephrine and vasopressin with lipid emulsion in a porcine model of asphyxial cardiac arrest after intravenous injection of bupivacaine. Anesth Analg. 2008;106(5):1566–71.
Berg RA, Henry C, Otto CW, Sanders AB, Kern KB, Hilwig RW, et al. Initial end-tidal CO2 is markedly elevated during cardiopulmonary resuscitation after asphyxial cardiac arrest. Pediatr Emerg Care. 1996;12(4):245–8.
Berg RA, Otto CW, Kern KB, Hilwig RW, Sanders AB, Henry CP, et al. A randomized, blinded trial of high-dose epinephrine versus standard-dose epinephrine in a swine model of pediatric asphyxial cardiac arrest. Crit Care Med. 1996;24(10):1695–700.
Berg RA, Hilwig RW, Kern KB, Babar I, Ewy GA. Simulated mouth-to-mouth ventilation and chest compressions (bystander cardiopulmonary resuscitation) improves outcome in a swine model of prehospital pediatric asphyxial cardiac arrest. Crit Care Med. 1999;27(9):1893–9.
Berg RA, Kern KB, Otto CW, Samson RA, Sanders AB, Ewy GA. Ventricular fibrillation in a swine model of acute pediatric asphyxial cardiac arrest. Resuscitation. 1996;33(2):147–53.
Iglesias JM, Lopez-Herce J, Urbano J, Solana MJ, Mencia S, Del Castillo J. Chest compressions versus ventilation plus chest compressions in a pediatric asphyxial cardiac arrest animal model. Intensive Care Med. 2010;36(4):712–6.
Jasani MS, Nadkarni VM, Finkelstein MS, Hofmann WT, Salzman SK. Inspiratory-cycle instillation of endotracheal epinephrine in porcine arrest. Acad Emerg Med. 1994;1(4):340–5.
Lopez-Herce J, Fernandez B, Urbano J, Mencia S, Solana MJ, del Castillo J, et al. Terlipressin versus adrenaline in an infant animal model of asphyxial cardiac arrest. Intensive Care Med. 2010;36(7):1248–55.
Voelckel WG, Lurie KG, McKnite S, Zielinski T, Lindstrom P, Peterson C, et al. Comparison of epinephrine and vasopressin in a pediatric porcine model of asphyxial cardiac arrest. Crit Care Med. 2000;28(12):3777–83.
Dickerson JWT, Dobbing J. Prenatal and postnatal growth and development of the central nervous system of the pig. Proc R Soc Lond. 1967;166:384–95.
Cerchiari EL, Hoel TM, Safar P, Sclabassi RJ. Protective effects of combined superoxide dismutase and deferoxamine on recovery of cerebral blood flow and function after cardiac arrest in dogs. Stroke. 1987;18(5):869–78.
Cerchiari EL, Sclabassi RJ, Safar P, Hoel TM. Effects of combined superoxide dismutase and deferoxamine on recovery of brainstem auditory evoked potentials and EEG after asphyxial cardiac arrest in dogs. Resuscitation. 1990;19(1):25–40.
Vaagenes P, Safar P, Moossy J, Rao G, Diven W, Ravi C, et al. Asphyxiation versus ventricular fibrillation cardiac arrest in dogs. Differences in cerebral resuscitation effects—a preliminary study. Resuscitation. 1997;35(1):41–52.
Ment LR, Stewart WB, Duncan CC, Pitt BR, Cole J. Beagle pup model of brain injury: regional cerebral blood flow and cerebral prostaglandins. J Neurosurg. 1987;67(2):278–83.
Nattie EE, Edwards WH, Marin-Padilla M. Newborn puppy cerebral acid-base regulation in experimental asphyxia and recovery. J Appl Physiol. 1984;56(5):1178–86.
Claesson A, Svensson L, Silfverstolpe J, Herlitz J. Characteristics and outcome among patients suffering out-of-hospital cardiac arrest due to drowning. Resuscitation. 2008;76(3):381–7.
Youn CS, Choi SP, Yim HW, Park KN. Out-of-hospital cardiac arrest due to drowning: an Utstein Style report of 10 years of experience from St. Mary’s Hospital. Resuscitation. 2009;80(7):778–83.
Ogawa T, Akahane M, Koike S, Tanabe S, Mizoguchi T, Imamura T. Outcomes of chest compression only CPR versus conventional CPR conducted by lay people in patients with out of hospital cardiopulmonary arrest witnessed by bystanders: nationwide population based observational study. BMJ. 2011;342:c7106.
Zhao H, Shimohata T, Wang JQ, Sun G, Schaal DW, Sapolsky RM, et al. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci. 2005;25(42):9794–806.
Sun S, Tang W, Song F, Yu T, Ristagno G, Shan Y, et al. The effects of epinephrine on outcomes of normothermic and therapeutic hypothermic cardiopulmonary resuscitation. Crit Care Med. 2010;38(11):2175–80.
Tortorici MA, Kochanek PM, Poloyac SM. Effects of hypothermia on drug disposition, metabolism, and response: a focus of hypothermia-mediated alterations on the cytochrome P450 enzyme system. Crit Care Med. 2007;35(9):2196–204.
Hobbs C, Thoresen M, Tucker A, Aquilina K, Chakkarapani E, Dingley J. Xenon and hypothermia combine additively, offering long-term functional and histopathologic neuroprotection after neonatal hypoxia/ischemia. Stroke. 2008;39(4):1307–13.
Laptook A, Tyson J, Shankaran S, McDonald S, Ehrenkranz R, Fanaroff A, et al. Elevated temperature after hypoxic–ischemic encephalopathy: risk factor for adverse outcomes. Pediatrics. 2008;122(3):491–9.
Wu X, Drabek T, Kochanek PM, Henchir J, Stezoski SW, Stezoski J, et al. Induction of profound hypothermia for emergency preservation and resuscitation allows intact survival after cardiac arrest resulting from prolonged lethal hemorrhage and trauma in dogs. Circulation. 2006;113(16):1974–82.
Vereczki V, Martin E, Rosenthal RE, Hof PR, Hoffman GE, Fiskum G. Normoxic resuscitation after cardiac arrest protects against hippocampal oxidative stress, metabolic dysfunction, and neuronal death. J Cereb Blood Flow Metab. 2006;26(6):821–35.
Kilgannon JH, Jones AE, Shapiro NI, Angelos MG, Milcarek B, Hunter K, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165–71.
Safar P, Abramson NS, Angelos M, Cantadore R, Leonov Y, Levine R, et al. Emergency cardiopulmonary bypass for resuscitation from prolonged cardiac arrest. Am J Emerg Med. 1990;8(1):55–67.
Eich C, Brauer A, Timmermann A, Schwarz SK, Russo SG, Neubert K, et al. Outcome of 12 drowned children with attempted resuscitation on cardiopulmonary bypass: an analysis of variables based on the “Utstein Style for Drowning”. Resuscitation. 2007;75(1):42–52.
Thiagarajan RR, Laussen PC, Rycus PT, Bartlett RH, Bratton SL. Extracorporeal membrane oxygenation to aid cardiopulmonary resuscitation in infants and children. Circulation. 2007;116(15):1693–700.
Suominen PK, Vallila NH, Hartikainen LM, Sairanen HI, Korpela RE. Outcome of drowned hypothermic children with cardiac arrest treated with cardiopulmonary bypass. Acta Anaesthesiol Scand. 2010;54(10):1276–81.
Trummer G, Foerster K, Buckberg GD, Benk C, Heilmann C, Mader I, et al. Successful resuscitation after prolonged periods of cardiac arrest: a new field in cardiac surgery. J Thorac Cardiovasc Surg. 2010;139(5):1325–32.
Wang JY, Shen J, Gao Q, Ye ZG, Yang SY, Liang HW, et al. Ischemic postconditioning protects against global cerebral ischemia/reperfusion-induced injury in rats. Stroke. 2008;39(3):983–90.
Incagnoli P, Ramond A, Joyeux-Faure M, Pepin JL, Levy P, Ribuot C. Erythropoietin improved initial resuscitation and increased survival after cardiac arrest in rats. Resuscitation. 2009;80(6):696–700.
Zhou Y, Fathali N, Lekic T, Ostrowski RP, Chen C, Martin RD, et al. Remote limb ischemic postconditioning protects against neonatal hypoxic–ischemic brain injury in rat pups by the opioid receptor/Akt pathway. Stroke. 2011;42(2):439–44.
Macias CA, Chiao JW, Xiao J, Arora DS, Tyurina YY, Delude RL, et al. Treatment with a novel hemigramicidin-TEMPO conjugate prolongs survival in a rat model of lethal hemorrhagic shock. Ann Surg. 2007;245(2):305–14.
Cook DJ, Teves L, Tymianski M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature. 2012;483(7388):213–7.
Sayre MR, O’Connor RE, Atkins DL, Billi JE, Callaway CW, Shuster M, et al. Part 2: evidence evaluation and management of potential or perceived conflicts of interest: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S657–64.
Venema AM, Groothoff JW, Bierens JJ. The role of bystanders during rescue and resuscitation of drowning victims. Resuscitation. 2010;81(4):434–9.
van Haeringen JR, Blokzijl EJ, van Dyl W, Kleine JW, Peset R, Sluiter HJ. Treatment of the respiratory distress syndrome following nondirect pulmonary trauma with positive end-expiratory pressure with special emphasis on near-drowning. Chest. 1974;66:30S–4S.
Eggink WF, Bruining HA. Respiratory distress syndrome caused by near- or secondary drowning and treatment by positive end-expiratory pressure ventilation. Neth J Med. 1977;20(4–5):162–7.
Pace NL. Positive end-expiratory pressure (PEEP) in treating salt water near-drowning. West J Med. 1975;122(2):165–7.
Rutledge RR, Flor RJ. The use of mechanical ventilation with positive end-expiratory pressure in the treatment of near-drowning. Anesthesiology. 1973;38(2):194–6.
Laurent I, Monchi M, Chiche JD, Joly LM, Spaulding C, Bourgeois B, et al. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol. 2002;40(12):2110–6.
Oddo M, Ribordy V, Feihl F, Rossetti AO, Schaller MD, Chiolero R, et al. Early predictors of outcome in comatose survivors of ventricular fibrillation and non-ventricular fibrillation cardiac arrest treated with hypothermia: a prospective study. Crit Care Med. 2008;36(8):2296–301.
Mullner M, Sterz F, Binder M, Hellwagner K, Meron G, Herkner H, et al. Arterial blood pressure after human cardiac arrest and neurological recovery. Stroke. 1996;27(1):59–62.
Sasser HC, Safar P. Arterial hypertension after cardiac arrest is associated with good cerebral outcome in patients. Crit Care Med. 1999;27(12):A29. (abstract).
Kaji AH, Hanif AM, Thomas JL, Niemann JT. Out-of-hospital cardiac arrest: early in-hospital hypotension versus out-of-hospital factors in predicting in-hospital mortality among those surviving to hospital admission. Resuscitation. 2011;82(10):1314–7.
Kilgannon JH, Roberts BW, Reihl LR, Chansky ME, Jones AE, Dellinger RP, et al. Early arterial hypotension is common in the post-cardiac arrest syndrome and associated with increased in-hospital mortality. Resuscitation. 2008;79(3):410–6.
de Pont AC, de Jager CP, van den Bergh WM, Schultz MJ. Recovery from near drowning and postanoxic status epilepticus with controlled hypothermia. Neth J Med. 2011;69(4):196–7.
Ponnusamy V, Beach RC, Blake J, Clarke P. A case of near-drowning: a case for routine cerebral monitoring. Acta Paediatr. 2010;99(3):463–6.
Krumholz A, Stern BJ, Weiss HD. Outcome from coma after cardiopulmonary resuscitation: relation to seizures and myoclonus. Neurology. 1988;38(3):401–5.
Celesia GG, Grigg MM, Ross E. Generalized status myoclonicus in acute anoxic and toxic-metabolic encephalopathies. Arch Neurol. 1988;45(7):781–4.
Hui AC, Cheng C, Lam A, Mok V, Joynt GM. Prognosis following Postanoxic Myoclonus Status epilepticus. Eur Neurol. 2005;54(1):10–3.
Arnoldus EP, Lammers GJ. Postanoxic coma: good recovery despite myoclonus status. Ann Neurol. 1995;38(4):697–8.
Morris HR, Howard RS, Brown P. Early myoclonic status and outcome after cardiorespiratory arrest. J Neurol Neurosurg Psychiatry. 1998;64(2):267–8.
Hovland A, Nielsen EW, Kluver J, Salvesen R. EEG should be performed during induced hypothermia. Resuscitation. 2006;68(1):143–6.
Sunde K, Dunlop O, Rostrup M, Sandberg M, Sjoholm H, Jacobsen D. Determination of prognosis after cardiac arrest may be more difficult after introduction of therapeutic hypothermia. Resuscitation. 2006;69(1):29–32.
Kaplan PW, Morales Y. Re: Status epilepticus: an independent outcome predictor after cerebral anoxia. Neurology. 2008;70(15):1295. (author reply 1295–1296).
Oksanen T, Skrifvars MB, Varpula T, Kuitunen A, Pettila V, Nurmi J, et al. Strict versus moderate glucose control after resuscitation from ventricular fibrillation. Inten Care Med. 2007;33(12):2093–100.
Hildebrand CA, Hartmann AG, Arcinue EL, Gomez RJ, Bing RJ. Cardiac performance in pediatric near-drowning. Crit Care Med. 1988;16(4):331–5.
Hein OV, Triltsch A, von Buch C, Kox WJ, Spies C. Mild hypothermia after near drowning in twin toddlers. Crit Care. 2004;8(5):R353–7.
Choi SP, Youn CS, Park KN, Wee JH, Park JH, Oh SH, et al. Therapeutic hypothermia in adult cardiac arrest because of drowning. Acta Anaesthesiol Scand. 2012;56(1):116–23.
Oakes DD, Sherck JP, Maloney JR, Charters AC III. Prognosis and management of victims of near-drowning. J Trauma. 1982;22(7):544–9.
Williamson JP, Illing R, Gertler P, Braude S. Near-drowning treated with therapeutic hypothermia. Med J Aust. 2004;181(9):500–1.
Varon J, Marik PE. Complete neurological recovery following delayed initiation of hypothermia in a victim of warm water near-drowning. Resuscitation. 2006;68(3):421–3.
Guenther U, Varelmann D, Putensen C, Wrigge H. Extended therapeutic hypothermia for several days during extracorporeal membrane-oxygenation after drowning and cardiac arrest two cases of survival with no neurological sequelae. Resuscitation. 2009;80(3):379–81.
Mizobuchi M, Nakamura S, Muranishi H, Utsunomiya M, Funatsu A, Kobayashi T et al. Hypothermia with extracorporeal membrane oxygenation for sudden cardiac death and submersion. Am J Emerg Med 2010;28(1):115, e111–114
Holzer M, Bernard SA, Hachimi-Idrissi S, Roine RO, Sterz F, Mullner M. Hypothermia for neuroprotection after cardiac arrest: systematic review and individual patient data meta-analysis. Crit Care Med. 2005;33(2):414–8.
Bernard SA, Rosalion A. Therapeutic hypothermia induced during cardiopulmonary resuscitation using large-volume, ice-cold intravenous fluid. Resuscitation. 2008;76(2):311–3.
Oddo M, Schaller MD, Feihl F, Ribordy V, Liaudet L. From evidence to clinical practice: effective implementation of therapeutic hypothermia to improve patient outcome after cardiac arrest. Crit Care Med. 2006;34(7):1865–73.
Busch M, Soreide E, Lossius HM, Lexow K, Dickstein K. Rapid implementation of therapeutic hypothermia in comatose out-of-hospital cardiac arrest survivors. Acta Anaesthesiol Scand. 2006;50(10):1277–83.
Belliard G, Catez E, Charron C, Caille V, Aegerter P, Dubourg O, et al. Efficacy of therapeutic hypothermia after out-of-hospital cardiac arrest due to ventricular fibrillation. Resuscitation. 2007;75:252–9.
Sunde K, Pytte M, Jacobsen D, Mangschau A, Jensen LP, Smedsrud C, et al. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73(1):29–39.
Arrich J. Clinical application of mild therapeutic hypothermia after cardiac arrest. Crit Care Med. 2007;35(4):1041–7.
Storm C, Steffen I, Schefold JC, Krueger A, Oppert M, Jorres A, et al. Mild therapeutic hypothermia shortens intensive care unit stay of survivors after out-of-hospital cardiac arrest compared to historical controls. Crit Care. 2008;12(3):R78.
Don CW, Longstreth WT Jr, Maynard C, Olsufka M, Nichol G, Ray T, et al. Active surface cooling protocol to induce mild therapeutic hypothermia after out-of-hospital cardiac arrest: a retrospective before-and-after comparison in a single hospital. Crit Care Med. 2009;37(12):3062–9.
Castrejon S, Cortes M, Salto ML, Benittez LC, Rubio R, Juarez M, et al. Improved prognosis after using mild hypothermia to treat cardiorespiratory arrest due to a cardiac cause: comparison with a control group. Rev Esp Cardiol. 2009;62(7):733–41.
Dumas F, Grimaldi D, Zuber B, Fichet J, Charpentier J, Pene F, et al. Is hypothermia after cardiac arrest effective in both shockable and nonshockable patients? Insights from a large registry. Circulation. 2011;123(8):877–86.
van der Wal G, Brinkman S, Bisschops LL, Hoedemaekers CW, van der Hoeven JG, de Lange DW, et al. Influence of mild therapeutic hypothermia after cardiac arrest on hospital mortality. Crit Care Med. 2011;39(1):84–8.
Azzopardi D, Robertson NJ, Cowan FM, Rutherford MA, Rampling M, Edwards AD. Pilot study of treatment with whole body hypothermia for neonatal encephalopathy. Pediatrics. 2000;106(4):684–94.
Debillon T, Daoud P, Durand P, Cantagrel S, Jouvet P, Saizou C, et al. Whole-body cooling after perinatal asphyxia: a pilot study in term neonates. Dev Med Child Neurol. 2003;45(1):17–23.
Jehle D, Meyer M, Gemme S Beneficial response to mild therapeutic hypothermia for comatose survivors of near-hanging. Am J Emerg Med 2010;28(3):390, e391–393
Nielsen N, Hovdenes J, Nilsson F, Rubertsson S, Stammet P, Sunde K, et al. Outcome, timing and adverse events in therapeutic hypothermia after out-of-hospital cardiac arrest. Acta Anaesthesiol Scand. 2009;53(7):926–34.
Dean JM, Kaufman ND. Prognostic indicators in pediatric near-drowning: the Glasgow coma scale. Crit Care Med. 1981;9(7):536–9.
Conn AW. Near-drowning and hypothermia. Can Med Assoc J. 1979;120(4):397–400.
Young GB, Doig GS. Continuous EEG monitoring in comatose intensive care patients: epileptiform activity in etiologically distinct groups. Neurocrit Care. 2005;2(1):5–10.
Rossetti AO. Treatment options in the management of status epilepticus. Curr Treat Options Neurol. 2010;12(2):100–12.
Carter BG, Butt W. Review of the use of somatosensory evoked potentials in the prediction of outcome after severe brain injury. Crit Care Med. 2001;29(1):178–86.
Rossetti AO. Novel anesthetics and other treatment strategies for refractory status epilepticus. Epilepsia. 2009;50(Suppl 12):51–3.
Rundgren M, Rosen I, Friberg H. Amplitude-integrated EEG (aEEG) predicts outcome after cardiac arrest and induced hypothermia. Intensive Care Med. 2006;32(6):836–42.
Wennervirta JE, Ermes MJ, Tiainen SM, Salmi TK, Hynninen MS, Sarkela MO, et al. Hypothermia-treated cardiac arrest patients with good neurological outcome differ early in quantitative variables of EEG suppression and epileptiform activity. Crit Care Med. 2009;37(8):2427–35.
Rossetti AO, Logroscino G, Liaudet L, Ruffieux C, Ribordy V, Schaller MD, et al. Status epilepticus: an independent outcome predictor after cerebral anoxia. Neurology. 2007;69(3):255–60.
Legriel S, Bruneel F, Sediri H, Hilly J, Abbosh N, Lagarrigue MH, et al. Early EEG monitoring for detecting postanoxic status epilepticus during therapeutic hypothermia: a pilot study. Neurocrit Care. 2009;11:338–44.
Monsalve F, Rucabado L, Ruano M, Cunat J, Lacueva V, Vinuales A. The neurologic effects of thiopental therapy after cardiac arrest. Intensive Care Med. 1987;13(4):244–8.
Paris PM, Stewart RD, Deggler F. Prehospital use of dexamethasone in pulseless idioventricular rhythm. Ann Emerg Med. 1984;13(11):1008–10.
Ichiba H, Yokoi T, Tamai H, Ueda T, Kim TJ, Yamano T. Neurodevelopmental outcome of infants with birth asphyxia treated with magnesium sulfate. Pediatr Int. 2006;48(1):70–5.
Acknowledgments
The authors are grateful to the Maatschappij tot Redding van Drenkelingen (Society to Rescue People from Drowning), Amsterdam, the Netherlands, which supported costs and organization of the consensus conference associated with this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
This manuscript is dedicated to the memory of Peter Safar, M.D. Peter Safar combined genius, incredible purpose, elegance, and humanism to move the collective fields of acute medicine to a new level. His work in resuscitation medicine, critical care, anesthesiology, emergency medicine, and disaster medicine saved countless lives. As a mentor he taught us a great deal. Peter left us with an important message—in both clinical care and research: namely, to always ask, “what is your intervention or research doing for the patient?” Whether physician or scientist, it is a critical message never to forget.
Portions of this manuscript are being reproduced in the Handbook of Drowning. Bierens JJ (ed.). Springer, 2nd Edition.
Rights and permissions
About this article
Cite this article
Topjian, A.A., Berg, R.A., Bierens, J.J.L.M. et al. Brain Resuscitation in the Drowning Victim. Neurocrit Care 17, 441–467 (2012). https://doi.org/10.1007/s12028-012-9747-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-012-9747-4