Abstract
Purpose
The purpose of this study was to compare the uptakes and diagnostic accuracies between 3′-deoxy-3′-[18F]fluorothymidine (FLT) and O-(2-[18F]fluoroethyl)-L-tyrosine (FET) PET in patients with a clinical suspicion of having a recurrence of glioma after multimodality treatment.
Methods
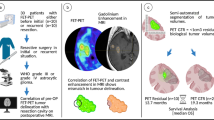
Thirty-two patients who underwent FLT and FET PET due to abnormal enhancement on magnetic resonance (MR) images were included. According to surgical confirmation or follow-up results, patients were divided into those with therapy-related benign changes (TRBCs) and those with recurrence. Recurrences were divided again into initial low-grade glioma (LGG) and high-grade glioma (HGG). The uptakes of FLT and FET were compared with the maximum standardized uptake value (SUVmax) and lesion-to-normal ratio (LNR). The diagnostic accuracies were compared via a receiver-operating-characteristic (ROC) curve analysis.
Results
The LNRs of FLT in recurrences with initial HGG (8.26 ± 5.02) were significantly higher than those in recurrences with initial LGG (3.43 ± 2.14) and TRBC (1.81 ± 0.60). The LNRs of FET in recurrence with initial HGG (2.70 ± 0.48) and LGG (3.03 ± 1.32) were significantly higher than those in the TRBC (1.60 ± 0.47). The areas under the ROC curve (AUCs) of FLT and FET for initial LGG were 0.768 and 0.893, respectively. The AUCs of FLT and FET for initial HGG were 1.000 and 0.964. However, there were no statistical significances. The results for comparing with SUVmax were the same as those with LNR.
Conclusions
Uptakes of FLT were different according to initial grade in patients with recurrent glioma, but those of FET were not. However, there were no statistical significances in the diagnostic accuracies according to initial grade between the two tracers in this study.




Similar content being viewed by others
References
Chen W (2007) Clinical applications of PET in brain tumors. J Nucl Med 48:1468–1481
Kaschten B, Stevenaert A, Sadzot B, Deprez M, Dequeldre C, Del Fiore G et al (1998) Preoperative evaluation of 54 gliomas by PET with fluorine-18-fluorodeoxyglucose and/or carbon-11-methionine. J Nucl Med 39:778–785
Goldman S, Levivier M, Pirotte B, Brucher JM, Wikler D, Damhaut P et al (1997) Regional methionine and glucose uptake in high-grade gliomas: a comparative study on PET guided stereotactic biopsy. J Nucl Med 38:1459–1462
Langleben DD, Segall GM (2000) PET in differentiation of recurrent brain tumor from radiation injury. J Nucl Med 41:1861–1867
Jager PL, Vaalburg W, Pruim J, de Viries EG, Langen KJ, Piers DA (2001) Radiolabeled amino acids: basic aspects and clinical applications in oncology. J Nucl Med 42:432–445
Wester HJ, Herz M, Weber W, Heiss P, Senekowitsch-Schmidtke R, Schwaiger M et al (1999) Synthesis and radiopharmacology of O-(2-[18F]fluoroethyl)-L-tyrosine for tumor imaging. J Nucl Med 40:205–212
Hamacher K, Coenen HH (2002) Efficient routine production of the 18F-labelled amino acid O-(2-[18F]fluoroethyl)-L-tyrosine. Appl Radiat Isot 57:853–856
Pauleit D, Floeth F, Hamacher K, Riemenschneider MJ, Reifenberger G, Müller HW et al (2005) O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with magnetic resonance imaging improves the diagnostic assessment of cerebral gliomas. Brain 128:678–687
Pöpperl G, Gotz C, Rachinger W, Gildehaus FJ, Tonn JC, Tatsch K (2004) Value of O-(2-[18F]fluoroethyl)-L-tyrosine PET for the diagnosis of recurrent glioma. Eur J Nucl Med Mol Imaging 31:1464–1470
Rachinger W, Goetz C, Pöpperl G, Gildehaus FJ, Kreth FW, Holtmannspötter M et al (2005) Positron emission tomography with O-(2-[18F]fluoroethyl)-L-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery 57:505–511
Floeth FW, Pauleit D, Sabel M, Stoffels G, Reifenberger G, Riemenschneider MJ et al (2007) Prognostic value of O-(2-18F-fluoroethyl)-L-tyrosine PET and MRI in low-grade glioma. J Nucl Med 48:519–527
Langen KJ, Hamacher K, Weckesser M, Floeth F, Stoffels G, Bauer D et al (2006) O-(2-[18F]fluoroethyl)-L-tyrosine: uptake mechanisms and clinical applications. Nucl Med Biol 33:287–294
Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC, Lawhorn-Crews JM et al (1998) Imaging proliferation in vivo with 18F-FLT and positron emission tomography. Nat Med 4:1334–1336
Choi SJ, Kim JS, Kim JH, Oh SJ, Lee JG, Kim CJ et al (2005) [18F]3′-deoxy-3′-fluorothymidine PET for the diagnosis and grading of brain tumors. Eur J Nucl Med Mol Imaging 32:653–659
Chen W, Cloughesy T, Kamdar N, Satymurthy N, Berqsneider M, Liau L et al (2005) Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J Nucl Med 46:945–952
Saga T, Kawashima H, Araki N, Takahashi JA, Nakashima Y, Hiqashi T et al (2006) Evaluation of primary brain tumors with FLT-PET: usefulness and limitations. Clin Nucl Med 31:774–780
Jacobs AH, Thomas A, Kracht LW, Li H, Dittmar C, Garlip G et al (2005) 18F-fluoro-L-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. J Nucl Med 46:1948–1958
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Di Chiro G, Oldfield E, Wright DC, De Michele D, Katz DA, Patronas NJ et al (1988) Cerebral necrosis after radiotherapy and/or intraarterial chemotherapy for brain tumors: PET and neuropathologic studies. AJR Am J Roentgenol 150:189–197
Valk PE, Budinger TF, Levin VA, Silver P, Gutin PH, Doyle WK (1988) PET of malignant cerebral tumors after interstitial brachytherapy: demonstration of metabolic activity and correlation with clinical outcome. J Neurosurg 69:830–838
Patronas NJ, DiChiro G, Brooks RA, DeLaPaz RL, Kornblith PL, Smith BH et al (1982) Work in progress: [18F]fluorodeoxyglucose and positron emission tomography in the evaluation of radiation necrosis of the brain. Radiology 144:885–889
Doyle WK, Budinger TF, Valk PE, Levin VA, Gutin PH (1987) Differentiation of cerebral radiation necrosis from tumor recurrence by [18F]FDG and 82Rb positron emission tomography. J Comp Assist Tomogr 11:563–570
Ricci PE, Karis JP, Heiserman JE, Fram EK, Bice AN, Drayer BP (1998) Differentiating recurrent tumor from radiation necrosis: time for re-evaluation of positron emission tomography? AJNR Am J Neuroradiol 19:407–413
Kahn D, Follett KA, Bushnell DL, Nathan MA, Piper JG, Madsen M et al (1994) Diagnosis of recurrent brain tumor: value of 201Tl SPECT vs. 18F-FDG PET. AJR Am J Roentgenol 163:1459–1465
Hustinx R, Pourdehnad M, Kaschten B, Alavi A (2005) PET imaging for differentiating recurrent brain tumor from radiation necrosis. Radiol Clin North Am 43:35–47
Chung JK, Kim YK, Kim SK, Lee YJ, Paek S, Yeo JS et al (2002) Usefulness of 11C-methionine PET in the evaluation of brain lesions that are hypo- or isometabolic on 18F-FDG PET. Eur J Nucl Med Mol Imaging 29:176–182
Yamamoto Y, Wong TZ, Turkington TG, Hawk TC, Reardon DA, Coleman RE (2006) 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography in patients with recurrent glioblastoma multiforme: comparison with Gd-DTPA enhanced magnetic resonance imaging. Mol Imaging Biol 8:340–347
Munch-Peterson B, Cloos L, Jensen K, Tyrsted G (1995) Human thymidine kinase 1. Regulation in normal and malignant cells. Adv Enzyme Regul 35:69–89
Rasey JS, Grierson JR, Wiens LW, Kolb PD, Schwwartz JL (2002) Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. J Nucl Med 43:1210–1217
Barthel H, Perumal M, Latigo J, He Q, Brady F, Luthra SK et al (2005) The uptake of 3'-deoxy-3’-[18F]fluorothymidine into L5178Y tumours in vivo is dependent on thymidine kinase 1 protein levels. Eur J Nucl Med Mol Imaging 32:257–263
Ljungdahl-Ståhle E, Guzenda E, Böttiger D, Wahren B, Oberq B, Ståhle L (1992) Penetration of zidovudine and 3′-fluoro-3′-deoxythymidine into the brain, muscle tissue, and veins in cynomolgus monkeys: relation to antiviral action. Antimicrob Agents Chemother 36:2418–2422
Stahle L, Borg N (2000) Transport of alovudine (3′-fluorothymidine) into the brain and the cerebrospinal fluid of the rat, studied by microdialysis. Life Sci 66:1805–1816
Stober B, Tanase U, Herz M, Seidl C, Schwaiger M, Senekowitsch-Schmidtke R (2006) Differentiation of tumour and inflammation: characterisation of [methyl-3H]methionine (MET) and O-(2-[18F] fluoroethyl)-L-tyrosine (FET) uptake in human tumour and inflammatory cells. Eur J Nucl Med Mol Imaging 33:932–939
Lahoutte T, Caveliers V, Camargo SM, Franca R, Ramadan T, Veljkovic E et al (2004) SPECT and PET amino acid tracer influx via system L (h4F2hc-hLAT1) and its transstimulation. J Nucl Med 45:1591–1596
Kracht LW, Friese M, Herholz K, Schroeder R, Bauer B, Jacobs A et al (2003) Methyl-[11C]-L-methionine uptake as measured by positron emission tomography correlates to microvessel density in patients with glioma. Eur J Nucl Med Mol Imaging 30:868–873
Bergstrom M, Ericson K, Hagenfeldt L, Mosskin M, von Holst H, Noren G et al (1987) PET study of methionine accumulation in glioma and normal brain tissue: competition with branched chain amino acids. J Comput Assist Tomogr 11:208–213
Nakamoto Y, Osman M, Cohade C, Marshall LT, Links JM, Kohlmyer S et al (2002) PET/CT: comparison of quantitative tracer uptake between germanium and CT transmission attenuation corrected images. J Nucl Med 43:1137–1143
Choi JY, Woo SK, Choi Y, Choe YS, Lee KH, Kim BT (2007) Quantitative comparisons between CT and 68Ge transmission attenuation corrected 18F-FDG PET images: measured attenuation correction vs. segmented attenuation correction. Nucl Med Mol Imaging 41:49–53
Bong JK, Kim HJ, Son HK, Park YY, Park HJ, Yun MJ et al (2005) Assessment of attenuation correction techniques with a 137Cs point source. Korean J Nucl Med 39:57–68
Bolender NF, Cromwell LD, Graves V, Margolis MT, Kerber CW, Wendling L (1983) Interval appearance of glioblastomas not evident in previous CT examinations. J Comput Assist Tomogr 7:599–603
Okamoto K, Ito J, Takahashi N, Ishikawa K, Furusawa T, Tokiguchi S et al (2002) MRI of high-grade astrocytic tumors: early appearance and evolution. Neuroradiology 44:395–402
Recht LD, Lew R, Smith TW (1992) Suspected low-grade glioma: is deferring treatment safe? Ann Neurol 31:431–436
Afra D, Osztie E, Sipos L, Vitanovics D (1999) Preoperative history and postoperative survival of supratentorial low-grade astrocytomas. Br J Neurosurg 13:299–305
Piepmeier JM, Christopher S, Spencer DD, Byrne T, Kim J, Knisel JP et al (1996) Variations in the natural history and survival of patients with supratentorial low-grade astrocytomas. Neurosurgey 38:872–878
Acknowledgements
This study was supported by the Korea Science and Engineering Foundation and Ministry of Education, Science and Technology, Republic of Korea through its National Nuclear Technology Program (Grant Code: M20702010002-08N0201-00200).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeong, S.Y., Lee, T.H., Rhee, C.H. et al. 3′-Deoxy-3′-[18F]fluorothymidine and O-(2-[18F]fluoroethyl)-L-tyrosine PET in Patients with Suspicious Recurrence of Glioma after Multimodal Treatment: Initial Results of a Retrospective Comparative Study. Nucl Med Mol Imaging 44, 45–54 (2010). https://doi.org/10.1007/s13139-009-0007-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-009-0007-2