Abstract
Otosclerosis is an otodystrophy of the otic capsule and is a cause of conductive, mixed or sensorineural hearing loss in the 2nd to 4th decades of life. Otosclerosis is categorised into two types, fenestral and retrofenestral. Imaging plays an important role in the diagnosis and management of otosclerosis. High-resolution CT (HRCT) of the temporal bone using 1-mm (or less) thick sections is the modality of choice for assessment of the labyrinthine windows and cochlear capsules. MRI has limited application in the evaluation of the labyrinthine capsules but is useful for assessment of the cochlear lumen prior to cochlear implantation in patients with profound hearing loss. The treatment of fenestral otosclerosis is primarily surgical with stapedectomy and prosthesis insertion. Patients with retrofenestral otosclerosis and profound hearing loss are treated medically using fluorides, but may derive significant benefit from cochlear implantation. This pictorial review aims to acquaint the reader with the pathology and clinical features of otosclerosis, the classical imaging appearances on CT and MRI, a radiological checklist for preoperative CT evaluation of otosclerosis, imaging mimics and a few examples of post-stapedectomy imaging and complications.
Teaching points
• Otosclerosis causes conductive, sensorineural and mixed hearing loss in adults.
• HRCT of the temporal bone is the diagnostic imaging modality of choice.
• Stapedectomy is used to treat fenestral otosclerosis.
• Fluorides and cochlear implantation are used to treat retrofenestral otosclerosis.
Similar content being viewed by others
Introduction
Otosclerosis is a unique autosomal dominant otodystrophy of the otic capsule. It is also called ‘otospongiosis’ as it is characterised by replacement of the normal ivory-like enchondral bone by spongy vascular bone. The decalcified foci tend to recalcify, becoming less vascular and more solid. Patients typically present in the 2nd- 4th decades of life with conductive hearing loss (CHL), sensorineural hearing loss (SNHL) or mixed hearing loss (MHL) and/or tinnitus. Otosclerosis is commoner in Caucasians as compared to blacks, Native Americans and Asians. The disease is more common in women and commonly bilateral (85 %). Otosclerosis is categorised into two types, fenestral and retrofenestral/cochlear. Retrofenestral otosclerosis rarely occurs without fenestral involvement; hence these manifestations are considered to be a continuum rather than two separate entities [1–4].
Fenestral Otosclerosis
Pathology, clinical findings and imaging
The more common fenestral type of otosclerosis involves the lateral wall of the bony labyrinth. Histologically, demineralised foci of spongy new bone typically occur in the region of the embryonic fissula ante fenestram, which is a cleft of fibrocartilagenous tissue between the inner and middle ear, just anterior to the oval window (Fig. 1). Bilateral involvement is common (Fig. 2). The promontory, round window niche and tympanic segment of the facial nerve canal can also be involved [1–3]. The disease gradually extends to involve the entire footplate of the stapes and may subsequently involve the cochlea. Heaped-up bony plaques formed in the healing phase typically cause narrowing of the oval and round windows. Involvement of the annular ligament leads to mechanical fixation of the stapedo-vestibular joint, which is responsible for the typical CHL/audiometric air-bone gap (Carhart’s notch) [1–5]. Complete obliteration of the oval window may occur in 2 % cases (Fig. 3). This rarely is associated with secondary torsional subluxation of the incus [2]. Otosclerosis can sometimes present as isolated round window involvement without pericochlear or oval window involvement [6].
The classical clinical findings include progressive CHL up to about 50–60 dB, absent stapedial reflexes, a normal tympanic membrane and no evidence of middle ear inflammation [1–5].
Imaging is usually not pursued in patients with uncomplicated CHL and characteristic clinical findings. The treatment of fenestral otosclerosis is primarily surgical with stapedectomy and stapes prosthesis insertion [1–5].
High-resolution CT (HRCT) of the temporal bone is the modality of choice for the preoperative evaluation of otosclerosis. Typically, very thin axial sections are obtained on a multidetector CT scanner, followed by axial and coronal reformats, respectively in the plane of and perpendicular to the lateral semicircular canal. If needed, additional reformats can be made, for example along the plane of the stapedial suprastructure. All studies are performed without contrast and the entire petrous temporal bone is included in the sections. Demineralised hypodense fenestral otosclerotic foci are best seen on axial HRCT because of the anteroposterior orientation of the oval window and stapes crura. Fenestral otosclerotic foci as small as 1 mm in size can be diagnosed on HRCT [1–5]. Some authors have mentioned a correlation between the size of the fenestral otosclerotic focus and the air-bone gap [5].
Apart from assessing the size and location of plaques and the narrowing of the oval window, the radiologist must evaluate the status of the round window, facial nerve canal, jugular bulb, middle ear cavity, ossicular chain and inner ear. Obliteration of the round window by the otosclerotic process may reduce the efficacy of stapedectomy and must be mentioned in the report (Fig. 4) [1–3]. Other or associated causes of CHL and SNHL must be ruled out prior to surgery. These include congenital ossicular fusion, ossicular discontinuity (Fig. 5), inflammatory middle ear disease (Fig. 6) and inner ear pathology such as acoustic neuroma and labyrinthitis ossificans [2]. Table 1 describes a recommended checklist for reporting preoperative HRCT of the temporal bone with clinical and surgical relevance of each of the points.
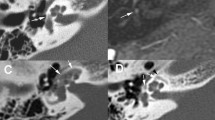
a Axial HRCT image of the right temporal bone in an adult patient with progressive right-sided CHL and remote history of ipsilateral head injury. A hypodense demineralised otosclerotic plaque (arrow) is noted in the fissula ante fenestram. b Axial HRCT image of the same patient as (a) at a slightly higher level. There is also evidence of malleo-incudal dislocation (arrow)
Axial (a) and coronal (b) HRCT images of the left temporal bone in an adult patient with left-sided CHL and previous history of left-sided otitis media. The soft-tissue density noted in the attic (asterisk) causing blunting of the scutum (arrow) is in favour of a cholesteatoma. In addition, a tiny hypodense fenestral otosclerotic focus (arrowheads) is noted anterior to the oval window
False-negative CT findings may occur in some cases of fenestral otosclerosis in the sclerotic phase when there are no irregularities of the bone contour [5]. The imaging differentials of fenestral otosclerosis are few. The cochlear cleft is a small non-osseous space in the otic capsule in the region of the fissula ante fenestram. It is a normal variant, commonly seen in children, and its incidence decreases with age. An inexperienced reader may mistake a cochlear cleft for a demineralised focus in the region of the fissula ante fenestram (Fig. 7) [2, 3, 7]. Tympanosclerois with post-inflammatory fixation of the stapes footplate may present clinically with identical CHL, especially with a healed tympanic membrane. This may cause a diagnostic dilemma in certain cases, although, this can be differentiated on CT by observing signs of inflammation in the middle ear and an underpneumatised mastoid [2, 3].
Post stapedectomy imaging and complications
Stapedectomy is commonly combined with insertion of a stapes prosthesis in order to restore ossicular chain continuity. The use of a radiodense stapes prosthesis helps radiological evaluation on HRCT (Fig. 8). Stapedectomy with prosthesis insertion is associated with some inherent complications. The common causes for recurrent CHL, vertigo and SNHL after stapes surgery include complete displacement of the prosthesis (Fig. 9), prosthesis displacement into the vestibule (Fig. 10), perilymphatic fistula, and development of reparative granulomas and labyrinthitis (Fig. 11) HRCT helps to evaluate the position of the prosthesis and rule out common complications. Additional MRI may act as an adjunct to rule out labyrinthitis ossicificans. MRI can rule out fibrotic changes in the labyrinth while ossifications are diagnosed exclusively by CT [2, 3, 8–10]. Repeat surgery may be mandatory for treatment of a dislocated prosthesis or closure of a perilymph fistula [8–10].
Para-coronal (a) and para-axial (b) HRCT images of the left temporal bone in a patient with persistent vertigo and left-sided SNHL after left stapedectomy. The stapes prosthesis (arrow) is well positioned. However a small sclerotic focus is seen within the vestibule (arrowheads) in keeping with labyrinthitis ossificans. A small fenestral otosclerotic focus (dashed arrow) is seen in the para-axial view
Retrofenestral or cochlear otosclerosis
Pathology, clinical findings and imaging
Retrofenestral or cochlear otosclerosis is much less common; however, it is nearly always associated with fenestral otosclerosis. Patients typically present with bilaterally symmetrical SNHL or MHL. Pulsatile tinnitus is also known to occur. Cochlear otosclerosis represents a continuum of the fenestral otosclerotic process. Histologically, foci of demineralised spongy vascular bone are seen in the cochlear capsule, which may extend around the vestibule, semicircular canals and internal auditory canal. The promontory may show a pink hue when seen through the tympanic membrane, called the Schwartze sign. Direct injury to the cochlea and spiral ligament due to the lytic process or release of proteolytic enzymes is implicated as a possible cause for the SNHL [1–4].
HRCT adequately demonstrates the demineralised foci in the otic capsule. The classical imaging appearance of cochlear otosclerosis on HRCT is a distinctive pericochlear hypodense double ring (which is also known as the 4th ring of Valvassori) (Fig. 12). Bilateral symmetry is common [1–4]. A CT grading of otosclerosis has been proposed by Symons/Fanning and is described in Table 2 [4]. Some authors mention that the severity of cochlear disease correlates with early onset as well as increasing severity of SNHL [4]. At times, MRI is performed prior to CT for assessing the cause of SNHL. A ring of pericochlear and perilabyrinthine intermediate signal on T1-weighted images and mild-moderate post-gadolinium enhancement has been reported in cochlear otosclerosis, more so in the active phase (Fig. 13) [2, 3, 11, 12]. MRI may also pick up other unassociated inner ear pathologies (Fig. 14). The HRCT appearance of cochlear otosclerosis is rarely mimicked by various diseases that demineralise the otic capsule, including osteogenesis imperfecta (Fig. 15) and Paget’s disease (Fig. 16). However the clinical manifestations and involvement of other bones suffice to differentiate these pathological conditions from cochlear otosclerosis [2–4, 11, 13].
a Axial CISS MR image of the skull base in an adult patient with left-sided SNHL. A small hypointense filling defect is seen in the left internal auditory canal (arrowhead), which may be suggestive of an acoustic neuroma. b Axial contrast-enhanced MR image of the same patient as (a), at the same level. The previously noted filling defect in the left internal auditory canal shows post-contrast enhancement, which indicates a small acoustic neuroma (arrowhead). There is also a suggestion of enhancement in the left pericochlear region and in the region of the left fissula ante fenestram (arrow), which suggests associated otosclerosis is present
New bone formation (membranous labyrinth ossification) is unusual with cochlear otosclerosis and is invariably limited to the basal turn of the cochlea [2, 14, 15]. This may be a relative contraindication for cochlear implantation (CI). In this setting, MRI is useful for assessment of the cochlear lumen (Fig. 17) [2, 3, 14, 15].
a Axial HRCT of the right temporal bone in a patient with known bilateral cochlear otosclerosis. There is almost complete obliteration of the basal turn of the right cochlea (arrow) by the otosclerotic process. b Axial MRI of the temporal bones in the same patient as (17a) at the level of the cochlea. There is significant obliteration of the fluid space in the basal turn of the right cochlea (arrow). The normal fluid space in the basal turn of the left cochlea (arrowhead) is shown for comparison
Treatment
Patients with cochlear otosclerosis are usually treated medically using fluorides [2, 3, 5, 11, 16]. Fluoride therapy may limit the growth of active otosclerotic foci and thereby prevent progression of SNHL [5]. However patients with bilateral profound SNHL may derive significant benefit from CI [2–4, 14, 15]. CI surgery in patients with otosclerosis may be challenging. There is high risk of partial insertion and misplacement of electrode arrays requiring revision surgery. This is ascribed to the ossification of the scala tympani in the basal turn of the cochlea [14, 15].
Conclusion
This article aims to serve as a concise review of the pathology and common imaging appearances of otosclerosis, imaging mimics and certain uncommon postoperative appearances and complications associated with otosclerosis.
Abbreviations
- HRCT:
-
high-resolution CT
- CHL:
-
conductive hearing loss
- SNHL:
-
sensorineural hearing loss
- MHL:
-
mixed hearing loss
- CI:
-
cochlear implantation
References
Valvassori GE (1993) Imaging of otosclerosis. Otolaryngol Clin North Am 26:359–371
Schwartz JD, Mukherji SK (2009) The Inner Ear and Otodystrophies. In: Swartz JD, Loevner LA (eds) Imaging of the Temporal Bone, 4th edn. Thieme, New York, pp 298–411
Hutchins T et al (2011) Otosclerosis. In: Harnsberger HR (ed) Diagnostic Imaging. Head and Neck, vol VI, 2nd edn. Amirsys, Manitoba, pp 4–40
Lee TC, Aviv RI, Chen JM, Nedzelski JM, Fox AJ, Symons SP (2009) CT Grading of otosclerosis. AJNR Am J Neuroradiol 30:1435–1439
Naumann IC, Porcellini B, Fisch U (2005) Otosclerosis: incidence of positive findings on high resolution computed tompography and their correlation to audiological test data. Ann Otol Rhinol Laryngol 114:709–716
Mansour S, Nicolas K, Ahmad HH (2011) Round window otosclerosis: radiologic classification and clinical correlations. Otol Neurotol 32:384–392
Chadwell JB, Halsted MJ, Choo DI, Greinwald JH, Benton C (2004) The cochlear cleft. AJNR Am J Neuroradiol 25:21–24
Ayache D, Lejeune D, Williams MT (2007) Imaging of postoperative senorineural complications of stapes surgery: a pictorial essay. Adv Otorhinolaryngol 65:308–313
Picuth D, Brandt S, Berghaus A, Spielmann RP, Heywang-Kobrunner (2000) Vertigo after stapes surgery: the role of high resolution CT. Br J Radiol 73:1021–1023
Kosling S, Bootz F (2001) CT and MR imaging after middle ear surgery. Eur J Radiol 40:113–118
Goh JPN, Chan LL, Tan TY (2002) MRI of cochlear otosclerosis. Br J Radiol 75:502–505
Ziyeh S, Berlin A, Ross UH, Reinhardt MJ, Schumacher M (1997) MRI of active otosclerosis. Neuroradiology 39:453–457
Alkhadi H, Rissmann D, Kollias SS (2004) Osteogenesis imperfecta of the temporal bone: CT and MR imaging in Van der Hoeve-de Kleyn syndrome. AJNR Am J Neuroradiol 25:1106–1109
Rotteveel LJ, Proops DW, Ramsden RT, Saeed SR, van Olphen AF, Mylanus EA (2004) Cochlear implantation in 53 patients with otosclerosis: demographics, computed tomographic scanning, surgery, and complications. Otol Neurotol 25:943–952
Ruckenstein MJ, Rafter KO, Montes M, Bigelow DC (2001) Management of far advanced otosclerosis in the era of cochlear implantation. Otol Neurotol 22:471–474
Causse JR, Causse JB, Uriel J, Berges J, Shambaugh GE Jr, Bretlau P (1993) Sodium fluoride therapy. Am J Otol 14:482–490
Acknowledgment
No grants were applied or received. No conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
Bela Purohit
• Study concept and design
• Manuscript preparation
• Literature search
• Manuscript revisions
Robert Hermans
• Guarantor of integrity of the study
• Study concept and design
• Manuscript editions and revisions
Katya Op de beeck
• Literature search
• Manuscript editions and revisions
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Purohit, B., Hermans, R. & Op de beeck, K. Imaging in otosclerosis: A pictorial review. Insights Imaging 5, 245–252 (2014). https://doi.org/10.1007/s13244-014-0313-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13244-014-0313-9