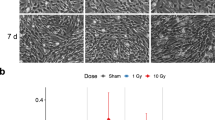
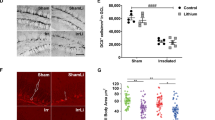
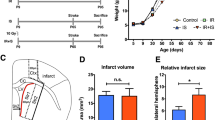
Abstract
In both pediatric and adult patients, cranial radiation therapy causes a debilitating cognitive decline that is poorly understood and currently untreatable. This decline is characterized by hippocampal dysfunction, and seems to involve a radiation-induced decrease in postnatal hippocampal neurogenesis. Here we show that the deficit in neurogenesis reflects alterations in the microenvironment that regulates progenitor-cell fate, as well as a defect in the proliferative capacity of the neural progenitor-cell population. Not only is hippocampal neurogenesis ablated, but the remaining neural precursors adopt glial fates and transplants of non-irradiated neural precursor cells fail to differentiate into neurons in the irradiated hippocampus. The inhibition of neurogenesis is accompanied by marked alterations in the neurogenic microenvironment, including disruption of the microvascular angiogenesis associated with adult neurogenesis and a marked increase in the number and activation status of microglia within the neurogenic zone. These findings provide clear targets for future therapeutic interventions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Strother, D.R. et al. Tumors of the central nervous system. in Principles and Practice of Pediatric Oncology, 4th edn. (eds. Pizzo, P.A. & Poplack, D.G.) 751–824 (Lipincott Williams and Wilkins, Philadelphia, Pennsylvania, 2002).
Surma-aho, O. et al. Adverse long-term effects of brain radiotherapy in adult low-grade glioma patients. Neurology 56, 1285–1290 (2001).
Roman, D.D. & Sperduto, P.W. Neuropsychological effects of cranial radiation: Current knowledge and future directions. Int. J. Radiat. Oncol. Biol. Phys. 31, 983–998 (1995).
Crossen, J.R., Garwood, D., Glatstein, E. & Neuwelt, E.A. Neurobehavioral sequelae of cranial irradiation in adults: A review of radiation-induced encephalopathy. J. Clin. Oncol. 12, 627–642 (1994).
Abayomi, O.K. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol. 35, 659–663 (1996).
Lee, P.W., Hung, B.K., Woo, E.K., Tai, P.T. & Choi, D.T. Effects of radiation therapy on neuropsychological functioning in patients with nasopharyngeal carcinoma. J. Neurol. Neurosurg. Psychiatry 52, 488–492 (1989).
Hodges, H. et al. Late behavioural and neuropathological effects of local brain irradiation in the rat. Behav. Brain Res. 91, 99–114 (1998).
Sienkiewicz, Z.J., Haylock, R.G. & Saunders, R.D. Prenatal irradiation and spatial memory in mice: Investigation of dose-response relationship. Int. J. Radiat. Biol. 65, 611–618 (1994).
Tada, E., Parent, J.M., Lowenstein, D.H. & Fike, J.R. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience 99, 33–41 (2000).
Peissner, W., Kocher, M., Treuer, H. & Gillardon, F. Ionizing radiation-induced apoptosis of proliferating stem cells in the dentate gyrus of the adult rat hippocampus. Brain Res. Mol. Brain Res. 71, 61–68 (1999).
Parent, J.M., Tada, E., Fike, J.R. & Lowenstein, D.H. Inhibition of dentate granule cell neurogenesis with brain irradiation does not prevent seizure-induced mossy fiber synaptic reorganization in the rat. J. Neurosci. 19, 4508–4519 (1999).
Snyder, J.S., Kee, N. & Wojtowicz, J.M. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J. Neurophysiol. 85, 2423–2431 (2001).
Kempermann, G., Kuhn, H.G. & Gage, F.H. More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495 (1997).
Gould, E., Beylin, A., Tanapat, P., Reeves, A. & Shors, T.J. Learning enhances adult neurogenesis in the hippocampal formation. Nature Neurosci. 2, 260–265 (1999).
Shors, T.J. et al. Neurogenesis in the adult is involved in the formation of trace memories. Nature 410, 372–376 (2001).
Dawirs, R.R., Hildebrandt, K. & Teuchert-Noodt, G. Adult treatment with haloperidol increases dentate granule cell proliferation in the gerbil hippocampus. J. Neural Transm. 105, 317–327 (1998).
Leventhal, C., Rafii, S., Rafii, D., Shahar, A. & Goldman, S.A. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol. Cell Neurosci. 13, 450–464 (1999).
Vallieres, L., Campbell, I.L., Gage, F.H. & Sawchenko, P.E. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J. Neurosci. 22, 486–492 (2002).
Calvo, W., Hopewell, J.W., Reinhold, H.S. & Yeung, T.K. Time- and dose-related changes in the white matter of the rat brain after single doses of X rays. Br. J. Radiol. 61, 1043–1052 (1988).
Sheline, G.E., Wara, W.M. & Smith, V. Therapeutic irradiation and brain injury. Int. J. Radiat. Oncol. Biol. Phys. 6, 1215–1228 (1980).
Pezner, R.D. & Archambeau, J.O. Brain tolerance unit: A method to estimate risk of radiation brain injury for various dose schedules. Int. J. Radiat. Oncol. Biol. Phys. 7, 397–402 (1981).
Marks, J.E., Baglan, R.J., Prassad, S.C. & Blank, W.F. Cerebral radionecrosis: Incidence and risk in relation to dose, time, fractionation and volume. Int. J. Radiat. Oncol. Biol. Phys. 7, 243–252 (1981).
Palmer, T.D., Willhoite, A.R. & Gage, F.H. Vascular niche for adult hippocampal neurogenesis. J. Comp Neurol. 425, 479–494 (2000).
Gundersen, H.J. et al. The new stereological tools: Dissector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS 96, 857–881 (1988).
Kuhn, H.G., Dickinson-Anson, H. & Gage, F.H. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J. Neurosci. 16, 2027–2033 (1996).
Gage, F.H. et al. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc. Natl. Acad. Sci. USA 92, 11879–11883 (1995).
Suhonen, J.O., Peterson, D.A., Ray, J. & Gage, F.H. Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature 383, 624–627 (1996).
Hong, J.H. et al. Induction of acute phase gene expression by brain irradiation. Int. J. Radiat. Oncol. Biol. Phys. 33, 619–626 (1995).
Heyser, C.J., Masliah, E., Samimi, A., Campbell, I.L. & Gold, L.H. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc. Natl. Acad. Sci. USA 94, 1500–1505 (1997).
Louissaint, A., Rao, S., Leventhal, C. & Goldman, S.A. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron 34, 945–960 (2002).
Lim, D.A. et al. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron 28, 713–726 (2000).
Seaberg, R.M. & van der Kooy, D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J. Neurosci. 22, 1784–1793 (2002).
Acknowledgements
We thank W. Stallcup for the NG2 antibody; B.E. Hoyte for help with the manuscript graphics; R. Malenka for the use of the Pritzker Foundation confocal microscope; D. Morhardt and D. Schaal for help in preparing the manuscript; and M. Brown, L. Fajardo and P. Fisher for valuable insights. This work was supported by grants MH20016-05 from the National Institute of Mental Health, R01CA76141 from the National Cancer Institute, R21NS40088 from the National Institute for Neurological Disorders and Stroke and Palmer Lab Startup funds from the Department of Neurosurgery, Stanford University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Monje, M., Mizumatsu, S., Fike, J. et al. Irradiation induces neural precursor-cell dysfunction. Nat Med 8, 955–962 (2002). https://doi.org/10.1038/nm749
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm749
This article is cited by
-
Comparative analysis of bevacizumab and LITT for treating radiation necrosis in previously radiated CNS neoplasms: a systematic review and meta-analysis
Journal of Neuro-Oncology (2024)
-
Irradiation and lithium treatment alter the global DNA methylation pattern and gene expression underlying a shift from gliogenesis towards neurogenesis in human neural progenitors
Translational Psychiatry (2023)
-
Neurocognitive considerations in the treatment of meningioma with radiation therapy: applications for quantitative neuroimaging and precision radiation medicine
Journal of Neuro-Oncology (2023)
-
Remote assessment of cognition and quality of life following radiotherapy for nasopharyngeal carcinoma: deep-learning-based predictive models and MRI correlates
Journal of Cancer Survivorship (2023)
-
Platelet-rich Plasma Improves Radiotherapy-induced Emotional Disorder and Cognitive Dysfunction, Neuroinflammation in Aged Rats by Inhibiting the Activation of NLRP3 Inflammasomes
Neurochemical Research (2023)