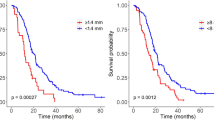
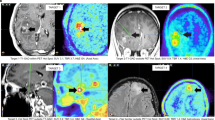
Abstract
Postoperative adjuvant radiation therapy and temozolomide chemotherapy have become the standard care for newly diagnosed malignant gliomas. The efficacy of these therapies has led to an increase in pseudoprogression and radiation necrosis, both of which are treatment-related effects whose appearance on standard MRI with gadolinium-based contrast agents resembles that of tumor progression or recurrence. Accurate diagnosis of these post-treatment lesions as either tumor recurrence or treatment effects (pseudoprogression or radiation necrosis) is important to determine the patient's prognosis. Modern advancements with magnetic resonance spectroscopy (MRS), diffusion-weighted imaging (DWI), and PET scans have shown promise for distinguishing tumor recurrence from treatment effects. Advances in radiographic techniques will become critically important with the emergence of new antiangiogenic therapies. Consequently, MRS, DWI, and PET need to be incorporated into routine post-treatment investigations to improve the specificity and sensitivity of distinguishing tumor recurrence from treatment effects. Further research will also be needed to develop improved algorithms that use these modalities, and to develop new modalities with even greater accuracy than those currently available.
Key Points
-
Temozolomide chemotherapy and radiotherapy have become the standard care for patients with malignant glioma
-
This therapeutic regimen has been associated with an increase in early, post-treatment, enhancing lesions on gadolinium-contrast MRI
-
These lesions are treatment effects, defined as either pseudoprogression (early radiographic changes) or radiation necrosis (later, subacute radiographic changes)
-
Pseudoprogression and radiation necrosis usually resolve spontaneously, although mass effects from radiation necrosis sometimes require surgical treatment; however, these changes are challenging to distinguish from tumor recurrence
-
Modern biologic and radiographic imaging techniques such as MRI, diffusion-weighted imaging, and magnetic resonance spectroscopy have shown potential in differentiating between tumor recurrence and radiation effects
-
Advances in radiographic and other imaging techniques used to detect tumors are needed; new antivascular therapies render standard contrast-enhanced MRI unreliable for assessing tumor recurrence
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 (2005).
Brandes, A. A. et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J. Clin. Oncol. 26, 2192–2197 (2008).
Mirimanoff, R. O. et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J. Clin. Oncol. 24, 2563–2569 (2006).
Brandsma, D., Stalpers, L., Taal, W., Sminia, P. & van den Bent, M. J. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 9, 453–461 (2008).
Chamberlain, M. C., Glantz, M. J., Chalmers, L., Van Horn, A. & Sloan, A. E. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J. Neurooncol. 82, 81–83 (2007).
de Wit, M. C., de Bruin, H. G., Eijkenboom, W., Sillevis Smitt, P. A. & van den Bent, M. J. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology 63, 535–537 (2004).
Zeng, Q. S. et al. Multivoxel 3D proton MR spectroscopy in the distinction of recurrent glioma from radiation injury. J. Neurooncol. 84, 63–69 (2007).
Kumar, A. J. et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 217, 377–384 (2000).
Mullins, M. E. et al. Radiation necrosis versus glioma recurrence: conventional MR imaging clues to diagnosis. AJNR Am. J. Neuroradiol. 26, 1967–1972 (2005).
Zeng, Q. S., Li, C. F., Liu, H., Zhen, J. H. & Feng, D. C. Distinction between recurrent glioma and radiation injury using magnetic resonance spectroscopy in combination with diffusion-weighted imaging. Int. J. Radiat. Oncol. Biol. Phys. 68, 151–158 (2007).
Rock, J. P. et al. Associations among magnetic resonance spectroscopy, apparent diffusion coefficients, and image-guided histopathology with special attention to radiation necrosis. Neurosurgery 54, 1111–1117 (2004).
Kashimura, H., Inoue, T., Beppu, T., Ogasawara, K. & Ogawa, A. Diffusion tensor imaging for differentiation of recurrent brain tumor and radiation necrosis after radiotherapy—three case reports. Clin. Neurol. Neurosurg. 109, 106–110 (2007).
Rachinger, W. et al. Positron emission tomography with O-(2-18F)-fluoroethyl)-L-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery 57, 505–511 (2005).
Herfarth, K. K., Gutwein, S. & Debus, J. Postoperative radiotherapy of astrocytomas. Semin. Surg. Oncol. 20, 13–23 (2001).
Nieder, C. et al. Radiotherapy for high-grade gliomas. Does altered fractionation improve the outcome? Strahlenther. Onkol. 180, 401–407 (2004).
Giglio, P. & Gilbert, M. R. Cerebral radiation necrosis. Neurologist 9, 180–188 (2003).
Ruben, J. D. et al. Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 65, 499–508 (2006).
Bauman, G. S. et al. Reirradiation of primary CNS tumors. Int. J. Radiat. Oncol. Biol. Phys. 36, 433–441 (1996).
Floyd, N. S. et al. Hypofractionated intensity-modulated radiotherapy for primary glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 58, 721–726 (2004).
Mayer, R. & Sminia, P. Reirradiation tolerance of the human brain. Int. J. Radiat. Oncol. Biol. Phys. 70, 1350–1360 (2008).
McDermott, M. W., Sneed, P. K. & Gutin, P. H. Interstitial brachytherapy for malignant brain tumors. Semin. Surg. Oncol. 14, 79–87 (1998).
Soffietti, R., Sciolla, R., Giordana, M. T., Vasario, E. & Schiffer, D. Delayed adverse effects after irradiation of gliomas: clinicopathological analysis. J. Neurooncol. 3, 187–192 (1985).
Kong, D. S. et al. Efficacy of stereotactic radiosurgery as a salvage treatment for recurrent malignant gliomas. Cancer 112, 2046–2051 (2008).
Perry, A. & Schmidt, R. E. Cancer therapy-associated CNS neuropathology: an update and review of the literature. Acta Neuropathol. 111, 197–212 (2006).
Peca, C. et al. Early clinical and neuroradiological worsening after radiotherapy and concomitant temozolomide in patients with glioblastoma: tumor progression or radionecrosis? Clin. Neurol. Neurosurg. 111, 331–334 (2008).
Forsyth, P. A. et al. Radiation necrosis or glioma recurrence: is computer-assisted stereotactic biopsy useful? J. Neurosurg. 82, 436–444 (1995).
Levin, V. A. et al. Phase II study of accelerated fractionation radiation therapy with carboplatin followed by PCV chemotherapy for the treatment of anaplastic gliomas. Int. J. Radiat. Oncol. Biol. Phys. 53, 58–66 (2002).
Wong, C. S. & Van der Kogel, A. J. Mechanisms of radiation injury to the central nervous system: implications for neuroprotection. Mol. Interv. 4, 273–284 (2004).
Brown, W. R. et al. Capillary loss precedes the cognitive impairment induced by fractionated whole-brain irradiation: a potential rat model of vascular dementia. J. Neurol. Sci. 257, 67–71 (2007).
Brown, W. R., Thore, C. R., Moody, D. M., Robbins, M. E. & Wheeler, K. T. Vascular damage after fractionated whole-brain irradiation in rats. Radiat. Res. 164, 662–668 (2005).
Li, Y. Q., Chen, P., Haimovitz-Friedman, A., Reilly, R. M. & Wong, C. S. Endothelial apoptosis initiates acute blood–brain barrier disruption after ionizing radiation. Cancer Res. 63, 5950–5956 (2003).
Tan, J., Geng, L., Yazlovitskaya, E. M. & Hallahan, D. E. Protein kinase B/Akt-dependent phosphorylation of glycogen synthase kinase-3β in irradiated vascular endothelium. Cancer Res. 66, 2320–2327 (2006).
Rodemann, H. P. & Blaese, M. A. Responses of normal cells to ionizing radiation. Semin. Radiat. Oncol. 17, 81–88 (2007).
Lin, T. et al. Role of acidic sphingomyelinase in Fas/CD95-mediated cell death. J. Biol. Chem. 275, 8657–8663 (2000).
Liao, W. C. et al. Ataxia telangiectasia-mutated gene product inhibits DNA damage-induced apoptosis via ceramide synthase. J. Biol. Chem. 274, 17908–17917 (1999).
Quarmby, S., Kumar, P. & Kumar, S. Radiation-induced normal tissue injury: role of adhesion molecules in leukocyte–endothelial cell interactions. Int. J. Cancer 82, 385–395 (1999).
Nordal, R. A., Nagy, A., Pintilie, M. & Wong, C. S. Hypoxia and hypoxia-inducible factor-1 target genes in central nervous system radiation injury: a role for vascular endothelial growth factor. Clin. Cancer Res. 10, 3342–3353 (2004).
Hovinga, K. E. et al. Radiation-enhanced vascular endothelial growth factor (VEGF) secretion in glioblastoma multiforme cell lines—a clue to radioresistance? J. Neurooncol. 74, 99–103 (2005).
Gupta, V. K. et al. Vascular endothelial growth factor enhances endothelial cell survival and tumor radioresistance. Cancer J. 8, 47–54 (2002).
Stegh, A. H., Chin, L., Louis, D. N. & DePinho, R. A. What drives intense apoptosis resistance and propensity for necrosis in glioblastoma? A role for Bcl2L12 as a multifunctional cell death regulator. Cell Cycle 7, 2833–2839 (2008).
Griebel, M. et al. Reversible neurotoxicity following hyperfractionated radiation therapy of brain stem glioma. Med. Pediatr. Oncol. 19, 182–186 (1991).
Watne, K., Hager, B., Heier, M. & Hirschberg, H. Reversible edema and necrosis after irradiation of the brain. Diagnostic procedures and clinical manifestations. Acta Oncol. 29, 891–895 (1990).
Hegi, M. E. et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 352, 997–1003 (2005).
Vredenburgh, J. J. et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin. Cancer Res. 13, 1253–1259 (2007).
Vredenburgh, J. J. et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J. Clin. Oncol. 25, 4722–4729 (2007).
Bi-weekly temozolomide plus bevacizumab for adult patients with recurrent glioblastoma multiforme. Clinical Trials.gov: a service of the US NIH [online], (2009).
Bevacizumab and temozolomide following radiation and chemotherapy for newly diagnosed glioblastoma multiforme. Clinical Trials.gov: a service of the US NIH [online], (2009).
Holash, J. et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc. Natl Acad. Sci. USA 99, 11393–11398 (2002).
Wachsberger, P. R. et al. VEGF-Trap in combination with radiotherapy improves tumor control in u87 glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 67, 1526–1537 (2007).
Sathornsumetee, S. et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J. Clin. Oncol. 26, 271–278 (2008).
Batchelor, T. T. et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11, 83–95 (2007).
Dings, R. P. et al. Scheduling of radiation with angiogenesis inhibitors Anginex and Avastin improves therapeutic outcome via vessel normalization. Clin. Cancer Res. 13, 3395–3402 (2007).
Winkler, F. et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6, 553–563 (2004).
Zhou, Q., Guo, P. & Gallo, J. M. Impact of angiogenesis inhibition by sunitinib on tumor distribution of temozolomide. Clin. Cancer Res. 14, 1540–1549 (2008).
Lai, A. et al. Phase II pilot study of bevacizumab in combination with temozolomide and regional radiation therapy for up-front treatment of patients with newly diagnosed glioblastoma multiforme: interim analysis of safety and tolerability. Int. J. Radiat. Oncol. Biol. Phys. 71, 1372–1380 (2008).
Du, R. et al. HIF-1α induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell 13, 206–220 (2008).
Norden, A. D. et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology 70, 779–787 (2008).
Kunkel, P. et al. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 61, 6624–6628 (2001).
Rubenstein, J. L. et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia 2, 306–314 (2000).
Pope, W. B., Lai, A., Nghiemphu, P., Mischel, P. & Cloughesy, T. F. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology 66, 1258–1260 (2006).
Dietrich, J., Norden, A. D. & Wen, P. Y. Emerging anti-angiogenic treatments for gliomas—efficacy and safety issues. Curr. Opin. Neurol. 21, 736–744 (2008).
Bruehlmeier, M., Roelcke, U., Schubiger, P. A. & Ametamey, S. M. Assessment of hypoxia and perfusion in human brain tumors using PET with 18F-fluoromisonidazole and 15O-H2O. J. Nucl. Med. 45, 1851–1859 (2004).
McPherson, C. M. & Warnick, R. E. Results of contemporary surgical management of radiation necrosis using frameless stereotaxis and intraoperative magnetic resonance imaging. J. Neurooncol. 68, 41–47 (2004).
Chan, Y. L., Yeung, D. K., Leung, S. F. & Chan, P. N. Diffusion-weighted magnetic resonance imaging in radiation-induced cerebral necrosis. Apparent diffusion coefficient in lesion components. J. Comput. Assist. Tomogr. 27, 674–680 (2003).
Hein, P. A., Eskey, C. J., Dunn, J. F. & Hug, E. B. Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am. J. Neuroradiol. 25, 201–209 (2004).
Asao, C. et al. Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. AJNR Am. J. Neuroradiol. 26, 1455–1460 (2005).
Verma, R. et al. Multiparametric tissue characterization of brain neoplasms and their recurrence using pattern classification of MR images. Acad. Radiol. 15, 966–977 (2008).
Schlemmer, H. P. et al. Proton MR spectroscopic evaluation of suspicious brain lesions after stereotactic radiotherapy. AJNR Am. J. Neuroradiol. 22, 1316–1324 (2001).
Rabinov, J. D. et al. In vivo 3 T MR spectroscopy in the distinction of recurrent glioma versus radiation effects: initial experience. Radiology 225, 871–879 (2002).
Schlemmer, H. P. et al. Differentiation of radiation necrosis from tumor progression using proton magnetic resonance spectroscopy. Neuroradiology 44, 216–222 (2002).
Weybright, P. et al. Differentiation between brain tumor recurrence and radiation injury using MR spectroscopy. AJR Am. J. Roentgenol. 185, 1471–1476 (2005).
Yang, D. et al. Cerebral gliomas: prospective comparison of multivoxel 2D chemical-shift imaging proton MR spectroscopy, echoplanar perfusion and diffusion-weighted MRI. Neuroradiology 44, 656–666 (2002).
Nelson, S. J. Multivoxel magnetic resonance spectroscopy of brain tumors. Mol. Cancer Ther. 2, 497–507 (2003).
McKnight, T. R. et al. Histopathological validation of a three-dimensional magnetic resonance spectroscopy index as a predictor of tumor presence. J. Neurosurg. 97, 794–802 (2002).
Gonen, O., Gruber, S., Li, B. S., Mlynarik, V. & Moser, E. Multivoxel 3D proton spectroscopy in the brain at 1.5 versus 3.0 T: signal-to-noise ratio and resolution comparison. AJNR Am. J. Neuroradiol. 22, 1727–1731 (2001).
Inglese, M. et al. Three-dimensional proton spectroscopy of deep gray matter nuclei in relapsing-remitting MS. Neurology 63, 170–172 (2004).
Tsuyuguchi, N. et al. Methionine positron emission tomography for differentiation of recurrent brain tumor and radiation necrosis after stereotactic radiosurgery in malignant glioma. Ann. Nucl. Med. 18, 291–296 (2004).
Terakawa, Y. et al. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J. Nucl. Med. 49, 694–699 (2008).
Floeth, F. W., Wittsack, H. J., Engelbrecht, V. & Weber, F. Comparative follow-up of enhancement phenomena with MRI and proton MR spectroscopic imaging after intralesional immunotherapy in glioblastoma—report of two exceptional cases. Zentralbl. Neurochir. 63, 23–28 (2002).
Brandes, A. A. et al. Temozolomide 3 weeks on and 1 week off as first-line therapy for recurrent glioblastoma: phase II study from Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Br. J. Cancer 95, 1155–1160 (2006).
Gonzalez, J., Kumar, A. J., Conrad, C. A. & Levin, V. A. Effect of bevacizumab on radiation necrosis of the brain. Int. J. Radiat. Oncol. Biol. Phys. 67, 323–326 (2007).
Torcuator, R. et al. Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J. Neurooncol. 94, 63–68 (2009).
Acknowledgements
We appreciate the editorial assistance with this manuscript provided by N. Huh. I. Yang, the first author, was partially supported by a University of California, San Francisco Clinical and Translational Scientist Training Research Award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Yang, I., Aghi, M. New advances that enable identification of glioblastoma recurrence. Nat Rev Clin Oncol 6, 648–657 (2009). https://doi.org/10.1038/nrclinonc.2009.150
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2009.150
This article is cited by
-
ABCC4 suppresses glioblastoma progression and recurrence by restraining cGMP-PKG signalling
British Journal of Cancer (2024)
-
Clinical applications and prospects of PET imaging in patients with IDH-mutant gliomas
Journal of Neuro-Oncology (2023)
-
Comparison of diagnostic value of 68 Ga-DOTATOC PET/MRI and standalone MRI for the detection of intracranial meningiomas
Scientific Reports (2021)
-
Distinct imaging patterns of pseudoprogression in glioma patients following proton versus photon radiation therapy
Journal of Neuro-Oncology (2021)
-
Targeting VPAC1 Receptors for Imaging Glioblastoma
Molecular Imaging and Biology (2020)