Abstract
Traumatic spinal cord injury (SCI) has devastating consequences for the physical, social and vocational well-being of patients. The demographic of SCIs is shifting such that an increasing proportion of older individuals are being affected. Pathophysiologically, the initial mechanical trauma (the primary injury) permeabilizes neurons and glia and initiates a secondary injury cascade that leads to progressive cell death and spinal cord damage over the subsequent weeks. Over time, the lesion remodels and is composed of cystic cavitations and a glial scar, both of which potently inhibit regeneration. Several animal models and complementary behavioural tests of SCI have been developed to mimic this pathological process and form the basis for the development of preclinical and translational neuroprotective and neuroregenerative strategies. Diagnosis requires a thorough patient history, standardized neurological physical examination and radiographic imaging of the spinal cord. Following diagnosis, several interventions need to be rapidly applied, including haemodynamic monitoring in the intensive care unit, early surgical decompression, blood pressure augmentation and, potentially, the administration of methylprednisolone. Managing the complications of SCI, such as bowel and bladder dysfunction, the formation of pressure sores and infections, is key to address all facets of the patient's injury experience.
Similar content being viewed by others
Introduction
Spinal cord injury (SCI) is defined as damage to the spinal cord (Fig. 1) that temporarily or permanently causes changes in its function. SCI is divided into traumatic and non-traumatic aetiologies1. Traumatic SCI occurs when an external physical impact (for example, a motor vehicle injury, fall, sports-related injury or violence) acutely damages the spinal cord, whereas non-traumatic SCI occurs when an acute or chronic disease process, such as a tumour, infection or degenerative disc disease, generates the primary injury.
a | The spinal cord itself is organized into grey matter (which contains neuronal cell bodies) and white matter (which contains myelinated axons). The white matter can be further subdivided into several ascending or descending tracts, which are composed of bundles of axons that originate from and project to specific regions in the brain and periphery. These tracts transmit specific information, such as sensory information (for example, temperature or itch) or motor information. Spinal nerve roots enter the spinal cord and either convey sensory information to the spinal cord (through the sensory or dorsal root) or convey motor information to the periphery (through the motor or ventral root). b | The vertebral column encircles the spinal cord in protective bone and ligament, which, in humans, is segmented into 7 cervical, 12 thoracic, 5 lumbar and 5 sacral vertebrae. Blood is supplied to the spinal cord by the spinal arteries, which are located anteriorly and posteriorly and branch to perfuse the spinal cord parenchyma. The spinal cord is also surrounded by a protective layer of cerebrospinal fluid contained within the pachymeninges. c–e | Each segmental region of the spinal cord (part c) innervates a specific region of the skin (part d), muscle (part e) or organ group. Damage to the spinal cord can result in partial or complete loss of function below the level of the injury. Note that part e describes the ‘key muscles’ as described in the International Standards for Neurological Classification of Spinal Cord Injury. Parts a–c are adapted with permission from Ref. 218, Macmillan Publishers Limited. Part d and part e are adapted with permission from the American Spinal Injury Association: International Standards for Neurological Classification of Spinal Cord Injury, revised 2011; Atlanta, GA, Revised 2011, Updated 2015.
In traumatic SCI, the primary insult damages cells and initiates a complex secondary injury cascade, which cyclically produces the death of neurons and glial cells, ischaemia and inflammation. This cascade is followed by changes in the organization and structural architecture of the spinal cord, including the formation of a glial scar and cystic cavities. The glial scar and cystic cavities, in combination with poor endogenous remyelination and axonal regrowth, mean that the spinal cord has a poor intrinsic recovery potential, such that SCI causes permanent neurological deficits.
SCIs have devastating physical, social and vocational consequences for patients and their families, and a loss of independence and persistently increased lifelong mortality rates are the hallmarks of SCI. Furthermore, the direct costs for the care of patients with SCI are staggering at US$1.1–4.6 million per patient over their lifetime, which underscores the role of prevention as the most important intervention we can deliver. For SCI that cannot be prevented, the development of effective treatments becomes crucially important2.
The past three decades have marked an exciting time for the field, as numerous neuroprotective and neuroregenerative therapies have been translated from preclinical studies into clinical trials. Although undoubtedly impressive, further progress will require a concerted effort to better understand the pathophysiological cascade of SCI, the limitations in translating data obtained from animal models and how to apply combinatorial treatments to this complex, multifaceted disease process.
This Primer provides new researchers with a succinct, up-to-date foundation on SCI. Discussed herein are key aspects of epidemiology, pathophysiology and patient presentation, relevant to both translational researchers and basic scientists. The Primer also provides an overview of important in-practice and upcoming therapeutic strategies, including medical, surgical and cell-based treatments, and concludes with the current outlook for patients and future directions of the field.
Epidemiology
Incidence and prevalence
Epidemiological data on SCI are often divided into traumatic and non-traumatic aetiologies, suggesting important epidemiological distinctions1. However, data are most often reported by individual national or provincial databases, which make generalizations between countries difficult. In addition, data are often retrospective and based on treatment codes or surgical procedures, which fail to capture the true incidence and prevalence of SCI.
The incidence of SCI varies worldwide3 (Fig. 2). Among developed regions, the incidence of traumatic SCI is higher in North America (39 cases per million individuals) than in Australia (16 cases per million individuals) or western Europe (15 cases per million individuals), owing to higher rates of violent crime and self-harm4. By comparison, the prevalence of non-traumatic SCI has been estimated as 1,227 cases per million individuals in Canada and 364 cases per million individuals in Australia; reliable data from other countries are not available5,6.
The annual incidence of spinal cord injuries varies depending on geographical region. Adapted with permission from Ref. 3, Dove Press.
Traumatic SCI occurs more commonly in males (79.8%) than in females (20.2%)7. The age profile of individuals with a traumatic SCI has a bimodal distribution; one peak is between 15 and 29 years of age and the second, smaller but growing peak is in those >50 years of age8,9. In the United States, the proportion of patients with traumatic SCI >60 years of age increased from 4.6% in 1970 to 13.2% in 2008 (Refs 10,11). This trend is continuing in parallel with the ageing population of the world7.
Traffic accidents are the primary cause of all traumatic SCIs in North America and accounted for 38% of injuries between 2010 and 2014, although this number is gradually declining7. Falls are typically the second-most common cause of traumatic SCIs and accounted for 31% of injuries between 2010 and 2014, followed by sports-related injuries, which account for 10–17% of traumatic SCIs9,11. High-energy impacts, such as traffic accidents and sport-related injuries, are more common in younger individuals, whereas low-energy impacts, such as falls, disproportionately occur in individuals >60 years of age, in whom underlying spinal degenerative changes, such as degenerative cervical myelopathy, are common9,11. Indeed, the incidence of cervical SCI for the general population (0.13 per thousand-years)12 is much lower than for patients with degenerative cervical myelopathy (12.33 per thousand-years)13. Overall, in the general population, traumatic SCI occurs most frequently at the level of the cervical spine (∼60%), followed by thoracic (32%) and lumbosacral (9%)7.
Mortality
Although the survival of patients with traumatic SCI has improved over time, patients continue to have mortality rates that exceed those of age-matched controls14. Estimates for acute in-hospital mortality range from 4% to 17%, then after hospital discharge, annual mortality rates remain persistently high, with 3.8% of patients dying in the first year after injury, 1.6% in the second year and then 1.2% for every year thereafter. The risk of mortality increases with more-severe injuries, higher injury levels (that is, cervical SCIs are associated with higher mortality than lumbar SCIs), increasing patient age, the presence of multisystem trauma and higher-energy injury mechanisms. Despite modern medical care, patients with traumatic SCI have a significantly reduced lifespan. For example, the life expectancy after SCI for an individual 40 years of age is lowered to 23 years after cervical level 5 (C5)–C8 injury, 20 years after C1–C4 injury and 8.5 years if they are ventilator dependent2.
Mechanisms/pathophysiology
Acute injury phase
Traumatic SCI is pathophysiologically divided into primary and secondary injuries and can be temporally divided into the acute (<48 hours), subacute (48 hours to 14 days), intermediate (14 days to 6 months) and chronic (>6 months) phases (Fig. 3). The initial traumatic event (that is, the primary injury) produces immediate mechanical disruption and dislocation of the vertebral column, which causes compression or transection of the spinal cord. This focal region of damage injures neurons and oligodendrocytes (that is, the myelinating cell type of the central nervous system (CNS)), disrupts the vasculature and compromises the blood–spinal cord barrier. Together, these events immediately initiate a sustained secondary injury cascade, which leads to further damage to the spinal cord and neurological dysfunction. This damage can often be in excess of that caused by the primary injury.
a | The initial mechanical trauma to the spinal cord initiates a secondary injury cascade that is characterized in the acute phase (that is, 0–48 hours after injury) by oedema, haemorrhage, ischaemia, inflammatory cell infiltration, the release of cytotoxic products and cell death. This secondary injury leads to necrosis and/or apoptosis of neurons and glial cells, such as oligodendrocytes, which can lead to demyelination and the loss of neural circuits. b | In the subacute phase (2–4 days after injury), further ischaemia occurs owing to ongoing oedema, vessel thrombosis and vasospasm. Persistent inflammatory cell infiltration causes further cell death, and cystic microcavities form, as cells and the extracellular architecture of the cord are damaged. In addition, astrocytes proliferate and deposit extracellular matrix molecules into the perilesional area. c | In the intermediate and chronic phases (2 weeks to 6 months), axons continue to degenerate and the astroglial scar matures to become a potent inhibitor of regeneration. Cystic cavities coalesce to further restrict axonal regrowth and cell migration. Republished with permission of AlphaMed Press, from Concise review: bridging the gap: novel neuroregenerative and neuroprotective strategies in spinal cord injury, Ahuja, C. S. & Fehlings, M., Stem Cells Transl Med. 5, 7, 2016, permission conveyed through Copyright Clearance Center, Inc.
Secondary cellular changes during the acute phase of injury, such as cell dysfunction and death, are caused by cell permeabilization, pro-apoptotic signalling and ischaemic injury due to the destruction of the microvascular supply of the spinal cord within minutes of injury15,16. In addition, blood vessel injury can cause severe haemorrhages, which can expose the cord to an influx of inflammatory cells, cytokines and vasoactive peptides. Indeed, increases in the level of pro-inflammatory cytokines, such as tumour necrosis factor (TNF) and IL-1β, are evident in the spinal cord within minutes of injury17. This parallels the arrival of inflammatory cells (such as macrophages, neutrophils and lymphocytes) into the spinal cord, which remain in the cord well beyond the subacute phase. The subsequent overwhelming inflammatory response in the acute and subacute phases of injury, combined with the disrupted blood–spinal cord barrier, progressively add to spinal cord swelling. Swelling can lead to further mechanical compression of the cord, which can extend for multiple spinal segments and worsen the injury.
Subacute injury phase
In the acute-to-subacute period, ischaemia and excitotoxicity contribute to a loss of intracellular and extracellular ionic homeostasis, with a key mediator of cell death being intracellular calcium dysregulation in both neurons and glia. Data from animal models indicate that a high intracellular calcium concentration activates calpains, which can cause mitochondrial dysfunction and cell death18,19. Furthermore, ongoing necrosis of neurons and glia due to ischaemia, inflammation and excitotoxicity releases ATP, DNA and potassium, which can activate microglial cells. Activated microglia, in addition to other inflammatory cells such as activated macrophages, polymorphonuclear cells and lymphocytes, infiltrate the injury site, where they propagate the inflammatory response and contribute to ongoing apoptosis of neurons and oligodendrocytes. Phagocytic inflammatory cells can clear myelin debris at the injury site, but can also induce further damage to the spinal cord through the release of cytotoxic by-products, including free radicals (for example, O2−, hydrogen peroxide and peroxynitrite). These reactive oxygen species cause lipid peroxidation, DNA oxidative damage and protein oxidation, which cause additional necrotic and delayed apoptotic cell death, further contributing to the harsh post-injury microenvironment20,21.
High levels of the neurotransmitter glutamate are released from dying neurons and astrocytes and are poorly reabsorbed by surviving astrocytes22,23. This causes NMDA (N-methyl-D-aspartate), AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) and kainate receptor overactivation, which, when combined with the loss of ATP-dependent ion pump functions and subsequent resultant sodium dysregulation, can lead to excitotoxic cell death24,25. Neuronal death due to excitotoxicity, as well as the other insults discussed above, cyclically propagates the secondary injury cascade19.
The impaired autoregulatory capacity of the injured cord vasculature, in addition to the systemic effects of SCI (such as hypotension and respiratory failure; see Clinical manifestations section), can contribute to ongoing ischaemia that persists for days to weeks after injury. Prolonged ischaemia contributes to further neuronal and glial (predominantly oligodendrocyte) cell death and the propagation of the injury. The multiple causes of cell death that occur during the acute and subacute phases of SCI can produce greater damage than the original primary injury and form the basis for the neuroprotective interventions (see below).
Intermediate–chronic injury phase
As the acute inflammatory response subsides, the spinal cord lesion evolves through dynamic intermediate through to chronic phases that are marked by attempts at remyelination, vascular reorganization, alterations in the composition of the extracellular matrix (ECM) and remodelling of neural circuits19.
Cystic cavitations. In humans, the overwhelming cell death and degeneration in the acute phase of injury promotes the ex vacuo (that is, loss of tissue volume) formation of cystic cavities, which contain extracellular fluid, thin bands of connective tissue and macrophages26. The cystic cavities coalesce to become a formidable barrier to directed axonal regrowth and are a poor substrate for cell migration27,28.
Glial scar. Studies using animal models have shown a perilesional zone around the cystic cavities, in which reactive astrocytes proliferate and tightly interweave their processes, creating an inhibitory mesh-like array. In the acute phase, signalling from activated microglia, astrocytes and macrophages causes the secretion of ECM proteins that are inhibitory to axonal growth, such as chondroitin sulfate proteoglycans (CSPGs), tenascin and NG2 proteoglycan (also known as chondroitin sulfate proteoglycan 4), which condense with astrocytes to form the glial scar. The glial scar potently restricts axon regeneration (that is, the repair or regrowth of existing neural pathways, or the development of de novo pathways) and anatomical plasticity by inhibiting neurite outgrowth29,30.
Oligodendrocyte progenitor cells that express NG2 proteoglycan migrate to the lesion site and associate with dystrophic axons (that is, swollen, injured axons that can be found in the damaged CNS). Pericytes also proliferate after SCI, giving rise to stromal cells that might deposit numerous ECM proteins31. Furthermore, fibroblasts can infiltrate the perilesional region, particularly after breaks in the glial layer that separates neural tissue from the meninges26, to replace the ECM with fibrous connective tissue and dense collagen deposits. Together, these ECM and cellular changes represent a marked physical and biochemical barrier to regeneration. However, not all aspects of the scar pose an inhibitory barrier32; complete attenuation of astrocytes in the glial scar results in impaired regeneration, as astrogliosis in the acute–subacute phases is responsible for isolating the injury site, to reduce the spread of cytotoxic molecules and inflammatory cells into adjacent, uninjured parenchyma33,34. Furthermore, perilesional astrocytes might provide local trophic support and promote neovascularization35. This dual role of the glial scar continues to be investigated.
Adult CNS myelin. Even if regenerative efforts are able to overcome spinal cord lesions, properties of the adult mammalian CNS can still limit neurite regrowth. For example, molecules present in myelin are potent inhibitors of axon regeneration, and several molecules released by degenerating oligodendrocytes can contribute to the failure of regeneration. These molecules include neurite outgrowth inhibitor A (Nogo-A), oligodendrocyte-myelin glycoprotein (OMgp) and myelin-associated glycoprotein (MAG), which can all bind to the Nogo receptor and p75 neurotrophin receptor (p75NTR; also known as TNF receptor superfamily member 16) to activate RHOA and Rho-associate protein kinase (ROCK), which causes growth cone collapse, neurite retraction and increases the risk of apoptosis36. CSPGs in the glial scar can also activate the Nogo receptor, in addition to the membrane-bound protein tyrosine phosphatase-θ (PTPθ), to trigger growth cone collapse via the RHO–ROCK pathway37. Interestingly, individual knockout of Nogo, MAG and OMgp showed limited effects on axon regeneration in vivo, potentially owing to the synergistic inhibitory activity of myelin-associated proteins and CSPGs on axonal regeneration38,39. This continues to be an area of active investigation.
Attempts at remyelination. Although severe SCI can destroy substantial portions of the spinal cord white matter, a surviving subpial rim of demyelinated axons can persist in rodent models of injury40. These neurons are susceptible to subsequent injury and progressive Wallerian degeneration (that is, an ordered process of axonal death)40,41. In theory, oligodendrocyte precursor cells can differentiate into mature oligodendrocytes and remyelinate these axons. However, remyelination requires a coordinated inflammatory response by macrophages, lymphocytes and astrocytes, and is inhibited by the presence of EphrinB3 in myelin debris42,43, as well as molecules within the glial scar36,44,45. This could lead to poor remyelination post-injury, which in turn impairs functional recovery46.
Endogenous attempts at repair
Contrary to historical dogma, endogenous mechanisms exist for at least partial regeneration of the injured spinal cord. CNS neurons exhibit both anatomical and synaptic plasticity, which might contribute to ongoing functional recovery for years after injury47,48. Furthermore, neural precursor cell pools, which are mostly found in the ependymal layers of the central canal, as well as widely distributed oligodendrocyte precursor cells, can generate neurons, oligodendrocytes and astrocytes (including reactive astrocytes)49,50. These cells might have both helpful and detrimental roles throughout the post-injury regenerative process. Exploiting these endogenous mechanisms by increasing the recruitment of pro-regenerative cells51, producing a microenvironment that is more conducive to cell migration and neurite outgrowth52, and/or shifting the balance towards pro-regenerative cell phenotypes53 are some of the exciting areas of ongoing research. These and other mechanisms can be supplemented by the neuroprotective and neuroregenerative strategies discussed later, but barriers to regeneration still exist, meaning additional therapies to remove or overcome these barriers are necessary.
Animal models
Animal models have contributed to our understanding of the pathophysiology of SCI and have been useful for the preclinical testing of new therapies. The ideal animal model should anatomically and pathophysiologically resemble human SCI, require minimal training, be inexpensive and produce consistent results. Rat models are the most commonly used for SCI research and are well established and inexpensive, and the injury response is similar to that observed in humans (including the production of cystic cavities, glial scar formation and changes in the ECM) (Box 1). However, differences in size, molecular signalling, anatomy and the recovery potential following SCI have made direct translation challenging54. Numerous therapies in SCI and other CNS fields (such as stroke) have, unfortunately, been ineffective when translated to humans from small animals, owing to their inherent biological differences. Large animal models, such as non-human primates, overcome some of these barriers, but substantial differences in cost and unique housing requirements make their use less common and even they are unable to exactly mimic human SCI55. However, larger animal models can form an important intermediary model to confirm results from rodents by providing relevant safety, biodistribution and technical feasibility data56,57. Furthermore, testing novel therapies in multiple species is an important approach to bolster preclinical evidence before commencing clinical trials, as is now recommended for stroke therapies58.
Diagnosis, screening and prevention
Clinical manifestations
Fractures of the spinal column are often described by their vertebral level, but the neurological injury is described by the spinal cord level at which the nerve roots emerge. The discrepancy between the two becomes increasingly apparent in the mid-to-low regions of the thoracic spinal cord, where a fracture at thoracic level 8 (T8) might cause a neurological SCI at T12 and a fracture at T12 might cause a SCI at sacral level 1 (S1).
The clinical manifestations of SCI depend on the level of neurological injury and the amount of preserved spinal cord tissue. SCI can result in the partial or complete loss of sensorimotor function below the level of the injury. Depending on the level of the injury, this can lead to compromised respiratory function (including hypercapnia, hypoxaemia and poor secretion clearance59,60); for example, injuries above C5 disrupt innervation to the diaphragm, injuries above T11 disrupt innervation to the intercostal chest muscles and injuries above lumbar level 1 (L1) can disrupt innervation to the abdominal muscles.
In addition to disruption of sensorimotor function, SCI can affect the sympathetic nervous system, as preganglionic sympathetic neurons originate in the spinal cord, between T1 and L2. SCI can reduce sympathetic outflow from the spinal cord, which results in a loss of basal vascular tone below the level of injury (Fig. 4). In addition, high thoracic or cervical injuries can lead to severe hypotension and bradycardia (that is, neurogenic shock, see below)61,62. The loss of innervation to secondary lymphatic organs (such as the spleen) can induce secondary immunodeficiency (also known as immune paralysis), which can increase the susceptibility to infections (for example, urinary tract infections and pneumonia)63. These systemic manifestations of CNS injury are the leading causes of early mortality in patients with SCI.
Injuries in the cervical and high thoracic cord can disrupt the sympathetic outflow (blue line) to the heart and the peripheral vascular system, while preserving baroreceptor inputs (red line) and parasympathetic output (green line). As a result, parasympathetic innervation to the heart dominates in patients with cervical and upper thoracic injuries, which causes bradycardia and decreased cardiac output. This is further compounded by the loss of peripheral muscular and vascular tone, which promotes a redistribution of blood to the periphery with reduced venous return. Consequently, patients often experience hypotensive symptoms, particularly with exertion or upright positioning. The parasympathetic–sympathetic imbalance can also allow unchecked reflex spinal sympathetic stimulation as a consequence of noxious triggers (such as bladder distension or pressure sores), which leads to sudden peripheral vasoconstriction and acute hypertension. As a response, parasympathetic outflow above the injury level increases, leading to vasodilation, headaches, sweating and sinus congestion. This dangerous acute syndrome is known as autonomic dysreflexia. S2–S4, sacral levels 2–4.
Spinal shock. Spinal shock is defined as a temporary clinical state of flaccid paralysis post-SCI, including the loss of motor, sensory, autonomic and reflex function at or below the level of injury. Spinal shock is commonly confused with neurogenic shock (which is a hypotensive state caused by loss of sympathetic outflow). Spinal shock can affect the accuracy of the initial neurological examination, which is used to define the severity of SCI. However, understanding when a patient no longer has spinal shock is problematic and has been the subject of controversy64. However, the theory to which most individuals subscribe describes spinal shock as a four-phase progression, from an initial stage of areflexia or hyporeflexia to the later stage of the return of deep tendon reflexes and hyper-reflexia.
Neurogenic shock. Hypotension post-SCI has several causes, including hypovolaemia secondary to blood loss, the distributive pooling of venous blood within paralysed atonic musculature and bradycardia. Hypotension can also be caused by vasodilation secondary to loss of sympathetic tone65,66, which produces neurogenic shock and is also typified by hypotension, bradycardia, wide pulse pressure and warm pink extremities. Neurogenic shock is most clinically relevant with a neurological level of injury above T6, as these injuries prevent central impulses reaching the mid-thoracic spinal cord, which is where the sympathetic splanchnic nerves (which have an important role in maintaining vascular tone) arise. Importantly, in injuries above T6, the sympathetic outflow to the cardiac pacemaker can also be affected. Neurogenic shock is estimated to occur in up to 20% of patients with cervical level injuries, and bradycardia occurs in nearly all patients with severe cervical injuries during the acute phase66,67.
Diagnosis
After any traumatic injury, first responders rapidly assess patients in the field and attempt resuscitation en route to the hospital. During this period, the advanced trauma life support protocols dictate initial care, which includes airway, breathing and circulation support, along with the immobilization of the potentially injured and unstable spinal column using a rigid cervical collar and backboard. Although individual hospital approaches vary, most patients with trauma will undergo a gross neurological examination (which includes a voluntary motor and sensory examination of each limb and a rectal examination) and spinal imaging (using, for example, X-ray or CT imaging) if a SCI is suspected. Concerns on clinical examination or early radiographic imaging are followed by advanced imaging and detailed neurological examinations (see below).
Imaging. Plain X-ray, CT and MRI are the most commonly used radiological tools when investigating damage to the spine and SCI. Anterior–posterior (AP) and lateral cervical spine X-ray, AP chest and AP pelvis X-rays are performed in the trauma room. Although not particularly sensitive for the identification of subtle fractures involving the cervical spine, X-rays are useful to detect gross fracture dislocation injuries that are often associated with SCI. It is essential to ensure the adequacy of any X-rays with visualization of the rostral half of the T1 vertebrae.
CT has largely supplanted X-ray for the diagnosis of bone injuries in patients with trauma68,69. With respect to the spine, some authors (namely, M.G.F.) currently perform, and recommend, a high-resolution fine-cut CT of the cervical to lumbar spine. CT angiography can also be performed to evaluate the bilateral vertebral arteries in certain cervical injuries. AOSpine has also developed subaxial cervical70 (C3–C7) and thoracolumbar71 (T1–L5) classification systems to standardize nomenclature of bony and ligamentous spinal injuries. These systems convey key information on the fracture pattern (for example, compression injury and translational injury), including adjacent structure involvement (for example, facets, ligaments and vertebral artery) with modifiers for neurological status (for example, incomplete SCI and complete SCI).
Although extremely sensitive for diagnosing a fracture or dislocation of the spine, CT is less effective at evaluating the integrity of soft tissue structures, such as intervertebral discs, ligaments, the spinal cord and nerve roots, but MRI is well suited for assessing these structures72. Specifically, when evaluating for ligament or vertebral disc injury, the T2-weighted short-tau inversion recovery (STIR) sequence enables the identification of injury-related oedema and tissue disruption. MRI can identify spinal cord transection and can evaluate for the presence of oedema and/or haemorrhage73.
The timing of MRI can be crucial with respect to the treatment of patients with SCI and cervical facet dislocation. MRI before closed reduction (that is, correcting the dislocation with the use of traction) enables the detection of disc herniation, which, if present, can lead to a deterioration in neurological status, although this has been disputed74,75. However, MRI can, depending on the institution, substantially delay time to decompression of the spinal cord and involves the additional transfer of a patient with a highly unstable spine. On the basis of the existing evidence, the most recent iteration of the American Association of Neurological Surgeons and Congress of Neurological Surgeons (AANS/CNS) guidelines for the management of cervical SCI recommends MRI before performing an open reduction (that is, realignment of the broken bones following surgery to expose the bones) or a closed reduction in an unconscious or uncooperative patient; if a disc herniation is identified, the guidelines recommend an anterior approach to remove the disc before reduction76.
The role of MRI is rapidly evolving, and advanced microstructural techniques that can quantify physiological changes at a cellular level and assess axon integrity (for example, diffusion tensor imaging), myelination (for example, myelin water transfer) and the presence of key metabolites related to ischaemia, cell loss or gliosis (for example, magnetic resonance spectroscopy)30,77 are likely to see increased integration in the care of patients with SCI.
Electrophysiology. Numerous electrophysiological studies have been evaluated for predicting outcome and for tracking and monitoring recovery over time after traumatic SCI. Electrophysiology is an attractive tool as it does not require the patient to be conscious or communicative. Several parameters have been studied (Box 2), which can be used to derive measures of physiological and anatomical function, such as conduction time to motor neurons, cortical and spinal inhibition, spinal cord excitability (such as the H-reflex) and sensory impairment, among others. Although interesting as a research tool, electrophysiological measurements have not consistently demonstrated added value in predicting outcome in awake and alert patients with SCI78. However, electrophysiological measurements might provide insight into the mechanisms underlying the functional recovery of the patient (for example, regeneration, plasticity or adaptation), which could be of benefit as the field develops, such as for patient selection for clinical trials79.
Classification of SCI. Perhaps the most significant advancement related to our ability to diagnose and classify SCI over the past few decades has been the development of the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI)80,81. The ISNCSCI has been uniformly adopted by SCI clinical communities and serves as the main measure of neurological outcome in clinical trials.
The ISNCSCI comprises three central neurological summary scores: American Spinal Injury Association (ASIA) motor score (which grades muscle power from each myotome (that is, a group of muscles innervated by one spinal nerve root), the ASIA sensory score (which assesses light touch and pinprick sensation in 28 dermatomes (that is, an area of skin innervated by one spinal nerve root) from C2 to S4 or S5) and the ASIA Impairment Scale grade (which is used to determine the grade of SCI and encompasses the extent of remaining sensorimotor function; Box 3)80. We recommend that ISNCSCI examination is performed at the time of acute hospital admission as soon as reasonably possible to serve as a baseline for comparison throughout follow-up.
The ISNCSCI has substantial evidence of both intra-rater and inter-rater reliability82,83 and correlates with other clinical, radiological and electrophysiological proxies for injury severity. Going forward, work is needed to better define the clinical relevance of sensorimotor changes on the ISNCSCI to establish how many points of gain are to be considered ‘clinically important’.
SCI syndromes
SCI patterns can broadly be defined as either complete or incomplete. A third category, discomplete, describes those with clinically complete injuries but persistent evidence of subclinical (for example, electrophysiological) brain–muscle connectivity84. For incomplete injuries, several patterns of neurological deficit are associated with SCI syndromes, including central cord syndrome, Brown-Séquard syndrome (also known as hemi-cord syndrome), anterior cord syndrome and posterior cord syndrome (Fig. 5).
The major descending motor tracts are in yellow and the major ascending sensory tracts are in blue (part a), as also depicted in Fig. 1a. The patterns of sensorimotor loss exhibited in patients with spinal cord injury (SCI) syndromes can be explained by damage to specific spinal cord tracts with sparing of other tracts. For example, the disproportionate motor impairment of the upper limbs compared with the lower limbs in patients with central cord syndrome (part b) might be explained by the complete, non-selective injury to the corticospinal tract (which is thought to transmit impulses related to fine hand and finger movements), but the preservation of the extrapyramidal tracts (which are thought to control gross leg and proximal arm movements). In addition, the different levels of sensorimotor, pain and temperature loss in patients with Brown-Séquard syndrome (that is, the contralateral pain and temperature loss are detected several levels below that of the ipsilateral sensorimotor loss) can be explained by the decussation of the lateral spinothalamic tract over several spinal segments (part c). Anterior cord syndrome (part d) results in complete motor paralysis due to damage to the corticospinal tract, loss of pain and temperature sensation secondary to damage of the spinothalamic tract, but preservation of light-touch sensation and proprioception (as the dorsal columns are generally preserved by this injury). Posterior cord syndrome (part e) results in the reverse, with loss of light touch and proprioception but preservation of motor function, and pain and/or temperature sensation.
Central cord syndrome is the most common incomplete SCI syndrome and accounts for 15–25% of traumatic SCIs85. Most commonly, central cord syndrome is diagnosed in elderly patients with pre-existing cervical spondylosis and stenosis who present after a fall that results in cervical hyperextension86. Central cord syndrome is characterized by a disproportionate motor impairment of the upper limbs, rather than the lower limbs, in addition to bladder dysfunction and varying degrees of sensory loss85.
Brown-Séquard syndrome is most commonly observed in individuals with penetrating traumatic SCI, secondary to gunshot and knife wounds. Deficits in patients with Brown-Séquard syndrome include loss of motor function, light touch, proprioception and vibration sensation ipsilateral to the injury, and loss of pain and temperature sensation contralateral to the injury87.
Anterior and posterior cord syndromes are rarely observed in isolation in the context of traumatic SCI, but are more frequent in patients with non-traumatic SCI of vascular aetiology87.
Prognosis
Neurological recovery. Neurological recovery in patients with SCI is typically observed within the first 6 months after injury, but continued improvements can be seen up to 5 years later88,89. The prognosis for neurological recovery is variable and depends primarily on the initial severity of neurological injury; a more severe degree of initial injury portends a worsened prognosis at 1 year90,91. The neurological level of injury can also determine neurological recovery; in general, thoracic injuries (particularly complete injuries) are associated with reduced potential for motor recovery compared with injuries in the cervical or lumbar spinal cord. This is thought to exist because neurological recovery is more difficult to clinically detect in the thoracic region92,93.
Functional outcomes, in particular, the ability to walk, are of interest to patients. In general, patients with ASIA Impairment Scale grade A injuries are generally predicted to have a <5% chance of walking 1 year post-injury, regardless of the neurological level of injury94. Ambulatory rates are substantially higher for patients with incomplete injuries, but are variable and depend on the initial level of neurological injury94.
Predicting neurological recovery. Several tools have been developed to predict neurological recovery in patients with SCI. One rule by van Middendorp et al.95 primarily relies on acute clinical examination features and can accurately predict long-term walking potential. Other tools, such as that developed by Wilson et al.96, use age, neurological examination and MRI features and can accurately predict the likelihood of long-term functional independence, and Pavese et al.97 have generated two simple models to predict urinary continence and complete bladder emptying at 1 year after injury using motor, sensory and spinal cord independent measure (SCIM) subscale scores. Each of these might function as useful tools in the future to help clinicians to estimate long-term prognosis in the acute setting.
Management
‘Time is spine’ has emerged as a central concept in the management of any patient with SCI. Similar to other acute CNS insults, functional neural tissue is progressively lost during the hours after SCI, making it crucially important to rapidly diagnose patients and implement neuroprotective interventions during the acute injury phase. These treatments have the potential to substantially alter the long-term functional recovery of patients and to provide meaningful improvements in quality of life (QOL). Management of patients with SCI is complex and involves multiple stages of care, which can often continue for years after the initial injury.
Prehospital and hospital care setting
For any patient with suspected spinal trauma and/or traumatic SCI, complete immobilization of the cranio–spinal axis should be maintained. In the prehospital setting, this should involve transport with the use of a rigid spine board and application of a cervical collar. After rapid transport to the hospital, precautions, including flat bedrest with a cervical collar, should be maintained until confirmation or restitution of spinal stability.
Current AANS/CNS SCI guidelines state that management of acute patients with SCI, particularly those with complete cervical injuries, should be performed in an intensive care unit (ICU) with continuous cardiac, haemodynamic and respiratory monitoring76. Indeed, the existing low-quality (that is, from non-randomized, retrospective observational studies) clinical evidence suggests that admission to an ICU, with the early identification and management of systemic complications of SCI (including hypoxia, hypotension, pulmonary dysfunction and cardiovascular instability), has a role in reducing secondary injury and facilitating improved recovery30,41. Care in the ICU is more important when considering concomitant injuries that can accompany SCI, including traumatic brain injury, intra-abdominal injury, thoracic injuries, pelvic or long bone fractures and facial trauma. In all cases, transfers of care should be expedited to reduce diagnosis and intervention times, and the transfer of patients to a specialized SCI care centre is recommended by AANS/CNS guidelines76.
Medical management
Haemodynamics. In the ICU, one of the most essential components of acute SCI management is the maintenance of adequate spinal cord perfusion, through the avoidance of systemic hypotension and support of mean arterial pressure. Hypotension is common post-SCI; on the basis of findings from predominantly retrospective clinical studies, the 2013 AANS/CNS SCI guidelines recommend avoiding systemic hypotension (maintaining a systolic blood pressure of <90 mmHg) and maintaining a mean arterial pressure between 85 and 90 mmHg for the first 7 days post-injury76,98. In addition, oxygen saturation should be maintained at ≥90% and prophylaxis to prevent deep venous thrombosis should be administered as soon as possible.
Methylprednisolone sodium succinate. Historically, the most contentious issue surrounding the medical management of SCI is the suitability of the administration of high-dose intravenous methylprednisolone sodium succinate (MPSS) in the acute phase of injury. In preclinical evaluations, MPSS showed substantial promise as a neuroprotective agent99–101. Subsequent clinical evaluation of MPSS led to the completion of three large randomized clinical trials (that is, the National Acute Spinal Cord Injury Studies (NASCIS)). The second NASCIS study probably had the largest impact on clinical practice102–104, and compared a high-dose 24-hour infusion of MPSS with placebo, or with naloxone. In the primary analysis, no significant difference in neurological recovery was observed between patients treated with MPSS or those who received placebo. However, in a secondary analysis (involving patients treated ≤8 hours post-SCI), MPSS administration resulted in a 5-point increase in ASIA motor scores at 6 months follow-up compared with placebo administration103. In a 2012 Cochrane review, data from two other confirmatory randomized studies using the same dose of MPSS were collated with the data from the second NASCIS; overall, administration of a high-dose 24-hour infusion of MPSS results in a 4-point increase in ASIA motor scores at long-term follow-up compared with no treatment or placebo105. Regarding adverse effects, weak trends towards an increased incidence of gastrointestinal haemorrhage and wound infection were noted with MPSS, but this did not achieve significance. In line with these findings, a large proportion of the spine surgery community began the routine administration of high-dose MPSS for patients with SCI arriving at the hospital within 8 hours of injury106. However, numerous criticisms of this practice, and of the supporting body of literature, have emerged over the years107. Namely, critics have pointed to the potential for increased complications, the use of subgroup analyses in the second NASCIS to prove effect, small positive effect sizes and methodological limitations in the two NASCIS II confirmatory trials, as arguments against the routine use of MPSS within 8 hours.
Balancing the available perspectives and evidence, the latest AANS/CNS SCI guidelines (that is, the 2013 guidelines) recommend against the use of MPSS for SCI, arguing that the evidence of harm is more consistent than the evidence of potential benefit76,108. However, the stance adopted by the authors of these guidelines has been somewhat controversial given that, despite no new prospective, randomized data on the topic in the interval, the 2002 version of the AANS/CNS SCI guidelines recommended a 24-hour administration of MPSS, started within the first 8 hours after injury, as a treatment option109. As noted in recent written commentary, as well as in debate presentations at recent international neurosurgery meetings, this change in recommendation has placed the clinician in a somewhat precarious position110,111. An upcoming 2017 AOSpine guideline in the Global Spine Journal will suggest a 24-hour infusion of MPSS be offered to patients within 8 hours of acute SCI as a treatment option. Ultimately, the authors of this review feel that decisions surrounding MPSS therapy should be left to the physician involved, balancing the potential for benefit with the potential for complications, given the characteristics of the presenting patient.
Decompressive surgery. Surgical intervention is an essential cornerstone of the acute treatment for patients with spinal trauma and acute SCI (Fig. 6). Overall, surgery aims to realign the spinal column, re-establish spinal stability and decompression (that is, relief of bony or ligamentous compression) of the spinal cord. Surgery typically involves open reduction and decompression paired with an instrumented fusion (for example, using implanted metal hardware) to stabilize the spinal column in an anatomical position. The extent of surgery is tailored to the anatomical site, as well as the severity and extent of injury.
Arrows mark the cervical level 5 (C5)–C6 where the injury is centred. a | Preoperative CT imaging demonstrates a severe C5–C6 fracture dislocation (arrow), with compromise of the central spinal canal. b | Preoperative MRI shows ongoing compression of the spinal cord (arrow) and a bright T2-weighted signal in the surrounding ligaments that is suggestive of disruption. c | Following surgery, including cervical traction, surgical decompression and instrumented fusion anterior and posterior metal hardware can be seen on the CT, and the restoration of appropriate spinal alignment. d | Successful decompression of the spinal cord can be seen on the postoperative MRI.
From a biological perspective, ongoing compression of the spinal cord is thought to exacerbate local spinal cord ischaemia, thereby potentiating secondary injury112,113. Thus, decompressing the spinal cord early after SCI should help to limit the zone of injury and improve clinical outcomes. Indeed, evidence from a systematic review and a meta-analysis of preclinical studies showed that a longer duration of spinal cord compression was typically associated with worsened outcomes (including neurobehavioural recovery and blood flow disturbances114. However, clinical class I randomized evidence supporting the efficacy of early surgical decompression is still lacking. That being said, several prospective, non-randomized studies have supported the safety and efficacy of surgical decompression, including one study that noted an increased odds of a ≥2 grade improvement in the ASIA Impairment Scale grade with early (within 24 hours) decompression compared with late (>24 hours) decompressive surgery in patients with cervical SCIs. In addition, data from this study showed a trend towards a reduced incidence of acute in-hospital complications in the early surgery group, but imbalances between the treatment groups might have influenced outcomes. Other studies have shown an association between early decompressive surgery and significantly greater improvements in ASIA motor scale recovery115; specifically, in patients with ASIA Impairment Scale grade A injuries (Box 3), reduced length of hospital stay, complication rates and health care costs116. In another study, very early decompression (≤8 hours) was associated with significant improvement in 1 year SCIM scores and ASIA Impairment Scale grades117. No international clinical guideline regarding the timing of decompressive surgery exists118. However, one guideline supported by AOSpine has recently been completed and will be published in the Global Spine Journal in early 2017.
Local complications
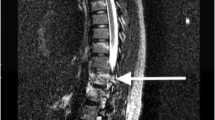
Syringomyelia. Post-traumatic syringomyelia occurs in ∼3% of patients with SCI and is characterized as a longitudinal fluid-filled cavity that can span many segments of the spinal cord and can lead to progressive myelopathy that occurs months to years after SCI (Fig. 7). Syringomyelia is distinct from the more common post-injury cystic cavitations, which are smaller and localized to the injury site. The pathophysiology of post-traumatic syringomyelia is not known but might involve a one-way valve that gradually leads to intraparenchymal cerebrospinal fluid and/or interstitial fluid accumulation119. Treatment depends on the clinical presentation and progression of symptoms120; asymptomatic patients are monitored with serial clinical and MRI examinations, whereas progressively, symptomatic patients might undergo surgical decompression by connecting the fluid cavity to the intrathecal space.
T2-weighted MRI of the cervical (parts a–c) and thoracic (parts d–f) spine in sagittal (part a and part d) and axial (parts b, c, e and f) planes shows a post-traumatic syrinx within the spinal cord parenchyma (white arrows) and kyphosis (that is, forward bending) of the thoracic spine at the initial site of the spinal cord injury (SCI; black arrow in part d). The syrinx extends well beyond the mid-thoracic site of the SCI into the high cervical spinal cord, which probably causes upper limb pain.
Neuropathic arthropathy. Neuropathic or Charcot joint arthropathy (that is, the slow progressive destruction of a joint) can lead to deformity, overlying skin ulceration and potentially fatal infections. The loss of sensation that is common after SCI allows repeated microtraumas to go unnoticed, which promotes bone resorption121. Furthermore, autonomic dysregulation can cause hyperaemia of denervated joints, which promotes further bone resorption. This arthropathy can occur in any joint, including the hips, knees, ankles, shoulders, elbows, spine and small joints.
Charcot arthropathy of the spine is often diagnosed 10–15 years after SCI and presents as deformity, paradoxical pain (below the sensory level of injury), a deterioration in neurological function and/or audible sounds with movement. Treatment might be conservative, such as clinical and radiographic follow-up, symptomatic (for example, treatment with analgesics or bracing) or surgical (such as vertebral fusion)122.
Spasticity. Spasticity is the velocity-dependent increase in muscle tone with exaggerated deep tendon reflexes that results from injury to upper motor neurons. Spasticity affects 65–78% of individuals with chronic SCI (>1 year post-injury) and can substantially affect mobilization, activities of daily living and sleep. Furthermore, spasticity can contribute to other local and systemic complications of SCI, including the development of pressure ulcers, contractures, fractures and cardiorespiratory deconditioning123. Treatment of spasticity may include physical therapy, systemic pharmacological treatments (for example, clonidine or γ-aminobutyric acid (GABA)-ergic drugs, such as diazepam and baclofen), intrathecal pharmacological treatments (for example, an intrathecal baclofen pump), local botulinum toxin injections or surgery (for example, tendon release surgery)123.
Systemic complications
Several chronic systemic complications can substantially affect the QOL of patients and their functional independence.
Cardiovascular. Analogous to changes observed during the acute period of injury, chronic cervical or thoracic SCI compromises sympathetic outflow from the CNS, which can lead to hypotension124 (Fig. 4). As a result, ∼60% of patients experience symptomatic orthostatic (or postural) hypotension (for example, dizziness, weakness and syncope)125. These symptoms occur consistently initially but gradually resolve over weeks to months, although they can persist for longer in some patients125. Treatment includes the use of lower extremity compression stockings, abdominal binding or medical management, including volume augmentation (such as the use of hydration, salt tablets or fludrocortisone) and/or peripheral vasoconstriction (for example, with midodrine, ephedrine or droxidopa)126.
Autonomic dysreflexia. Autonomic dysreflexia is an urgent condition that most commonly occurs in patients with injuries at or above T6 (particularly, in those with complete injuries). Autonomic dysreflexia is caused by the presence of a noxious stimulus below the level of injury (such as bladder distension, bowel impaction or pressure sores), which causes a reflex overstimulation of spinal sympathetic neurons, leading to vasoconstriction and dangerous acute hypertension127. As a response, parasympathetic outflow increases above the injury level and sympathetic outflow can be inhibited, depending on the injury level, which leads to vasodilation, headache, sweating and sinus congestion. Prompt treatment requires upright positioning of the patient, removal of the triggering stimulus and pharmacological anti-hypertensives for refractory cases128. Episodes of life-threatening autonomic dysreflexia can occur in both acute and chronic stages of injury, making long-term prevention key by avoiding noxious stimuli (for example, by frequent bowel and bladder care and repositioning to avoid pressure sores).
Respiratory. Paralysis of the phrenic nerve, intercostal muscles and/or abdominal muscles leads to reduced lung capacity, ineffective cough and accelerated fatigue with respiratory demand129. As a consequence, patients experience recurrent pneumonia, atelectasis (that is, alveolar collapse) and pleural effusion (fluids around the lungs), and are more likely to have sleep apnoea and respiratory failure130. Whereas long-term rehabilitation, which promotes cardiorespiratory conditioning, may be beneficial, the respiratory defects themselves restrict rehabilitation capacity and long-term independence. Owing to this, respiratory complications are the leading cause of mortality in patients with chronic SCI. In individuals with high cervical injuries, or those with poor respiratory reserve, lifelong ventilator dependency can also result131,132.
Secondary immunodeficiency. As previously mentioned, the disruption of CNS input to immune organs can result in the systemic dysfunction of macrophages, T cells, B cells and natural killer cells in a process known as immune paralysis. The clinical manifestation of this is an increased susceptibility to infections, such as pneumonia, urinary tract infections and wound infections133,134. Although the cause of immune paralysis continues to be investigated, cervical or high thoracic injuries have been shown to cause interruption of the sympathetic innervation of lymphatic organs and are associated with rapid splenic atrophy133. No accepted management for secondary immunodeficiency exists.
Genitourinary and gastrointestinal. Dysfunction of the genitourinary and gastrointestinal systems increases care requirements, the risk of infection and can be a source of substantial social and psychological stress in patients with SCI. Injuries at or above L1–L2 interrupt innervation of the detrusor, or the bladder muscle, and urinary sphincters, which can cause an inability to empty the bladder, acontractile bladder, urinary incontinence and recurrent infections135,136. Management includes urethral catheterization every few hours, the surgical creation of a urinary stoma, injections of botulinum toxin and pharmacological therapies (such as anticholinergics or α-blockers)124.
The neurological level of injury can also substantially affect sexual function. For example, injuries above T11 can affect psychogenic arousal (that is, erection or vaginal lubrication as a result of arousal in the brain) with preservation of reflexive arousal (that is, erection or vaginal lubrication as a result of genital stimulation) and the ability to orgasm. Conus injuries (that is, injuries in the sacral segment) can interfere with reflexive arousal but preserve psychogenic arousal. T12–L2 injuries with sacral segment sparing can preserve all sexual functions137.
Approximately 39% of patients with SCI report that bowel dysfunction significantly reduces their QOL124. SCI can interrupt the voluntary control of the anal sphincter (causing faecal retention) and/or the parasympathetic innervation of the bowel (in patients with lumbosacral injury). Both cases lead to constipation, an increased risk of infection and stress for patients. Treatments can range from dietary fibre intake, digital rectal stimulation or disimpaction and the use of suppositories, to implantation of an electrical stimulator or colostomy138,139.
Pressure sores. Pressure sores cause pain, increased care requirements and can be life-threatening if not promptly treated. Sores most commonly occur on the buttocks (31%), lateral thighs (26%), sacrum (18%), feet (7%) and ankles (4%)124. Prevention of pressure sores requires daily inspection and cleaning of the skin, but also a relief of the pressure on each region every few hours. Once a sore develops, diligent aseptic technique, debridement, dressing and nutritional support are vital to halt progression to life-threatening and limb-threatening infections140.
Neurogenic heterotopic ossification. Approximately 10–53% of patients with chronic SCI form ectopic bone in the connective tissue around joints, in a process called neurogenic heterotopic ossification. This ossification occurs most commonly in the large joints (for example, the hips, knees, elbows or shoulders), tends to develop within months of the SCI and presents with localized pain, redness, low-grade fever and increased spasticity141. The exact cause of neurogenic heterotopic ossification is not known, but it might involve a combination of local, humoral and neuro-immunological factors. Management can include physical therapy, pharmacological therapy (such as bisphosphonates and/or NSAIDs), radiation therapy or surgical resection of the ossification142.
Neuropathic pain. Neuropathic pain is experienced by up to 40% of patients with chronic SCI, has a mean onset of 1.2 years after injury and can have a substantial effect on the psychological well-being and QOL of patients. The mechanism underlying injury-level pain is thought to be sprouting of spinal cord fibres around damaged nerve roots, leading to inappropriate activation of primary afferent fibres and the initiation of pain by normally non-noxious stimuli (that is, allodynia). Below-injury-level pain is hypothesized to occur owing to a loss of spinal and supraspinal inhibitory signalling combined with the potentiation of brain pain-responsive areas. Neuropathic pain can be treated pharmacologically (for example, using antidepressants, anticonvulsants and/or opioids), surgically (such as implanted spinal cord stimulators, deep brain stimulators and dorsal root entry zone lesioning) or through non-allopathic treatments (such as acupuncture, massage and cognitive–behavioural therapy)143.
Rehabilitation
Rehabilitation requires an interdisciplinary approach involving nurses, physicians, dieticians, psychologists, physiotherapists, social workers, recreation therapists, speech therapists, orthotists and child life specialists. Rehabilitation can have significant effects on long-term health by helping patients to recover as much function as possible, prevent secondary complications, understand the extent of their injuries, cope with loss of independence and address other practical challenges, such as vocational and financial concerns.
Physical rehabilitation is focused on regaining function, enhancing any remaining function and preventing complications. Key components of rehabilitation are strength training, cardiovascular-focused exercise, respiratory conditioning, transfer or mobility training and stretching to prevent muscle contractures (that is, the permanent shortening of muscle). The patient's progress helps to dictate the level of ongoing care that is needed in the community and the use of assistive devices for daily living. Further high-quality (that is, level 1–2) trials of physical rehabilitation are required to validate the intuitive efficacy and compare specific treatment modalities144. Interestingly, physical rehabilitation can induce significant changes in cellular signalling and growth factor expression145. Early mobilization increases endogenous growth factor levels (such as insulin-like growth factor 1) and axon regeneration in animal models145. However, in clinical practice, ventilator dependence, poor vascular tone, neuropathic and somatic pain, psychosocial challenges and resource limitations in acute care institutions can make early mobilization challenging30. These important clinical barriers are often overlooked but represent formidable overarching challenges to recovery.
Weight-supported locomotor training (WSLT) uses assisted devices (such as Hocoma's Lokomat and HealthSouth's AutoAmbulator) and therapists to dynamically support the patient's weight while they attempt locomotion on a treadmill or open ground. The therapy seeks to enhance the remaining connectivity between regions above the SCI and the locomotor central pattern generator (that is, a region of neurons that, when activated, can initiate locomotion in the absence of sensory input or input from the brain) with the spinal cord. WSLT has been shown to improve assisted mobility, cardiorespiratory status and to prevent pressure sores and joint-related complications of SCI. A randomized, single-blind trial (n = 146) comparing 12 weeks of WSLT versus similar intensity physical rehabilitation found no significant difference in outcomes, but both groups had improvements in locomotion at 6 months, highlighting the importance of intensive rehabilitation146.
Occupational therapy focuses on integrating adaptive devices into people's daily lives to maximize functional independence at home and at work. Devices might include wheelchairs, lifts, braces, orthoses, environmental control units (such as lights, television or phones), bathroom equipment (such as showering or toileting), vehicle modifications for driving and others147. The US Department of Health and Human Services maintains a database (www.AbleData.com) of accessibility devices to help inform patients.
Functional electrical stimulation
Functional electrical stimulation (FES) uses small pulses of current to activate muscles and has been successfully used in the upper extremities for eating, gripping and writing. In the lower extremities, FES has been connected to a wheeled walker for ambulation (for example, the Parastep by Sigmedics, Inc.) and to stationary bicycles (for example, ERGYS 3 by Therapeutic Alliances, Inc. and RT300 by Restorative Therapies). FES can also be surgically implanted with electrodes on the anterior sacral nerve roots to provide patients with controllable bowel or bladder function. Typically, the implanted sacral nerve stimulator, such as the Vocare Bladder System (Finetech Medical), requires surgical lesioning of dorsal sensory roots to improve continence, but an open-label pilot study is under way to assess the system in patients with SCI without nerve sacrifice, with results expected by 2018 (NCT02978638). Importantly, FES can also enhance neuroplasticity and decrease the systemic complications of chronic SCI in patients148. In addition, FES-based exercises can double the oxygen uptake, triple the ventilation rate and improve the overall muscle to fat ratio in the body149,150. FES is an actively researched field, with the next generation of devices integrating more-advanced closed-loop feedback systems, greater MRI compatibility and novel stimulation programmes to reduce adverse effects and improve efficacy151.
Quality of life
QOL in patients with SCI is most often defined by the ability of patients to be independent of assisted care and to hold meaningful employment152. The most frequently used subjective measure of QOL is the Satisfaction with Life Scale (SWLS), and the most commonly used objective measure is the 36-Item Short-Form Health Survey (SF-36)153. A new scale to assess QOL in patients with SCI is the SCI-QOL, a patient-reported outcome measure comprising 18 domains, including metrics for physical, emotional and social aspects154.
Factors associated with QOL
Patients with SCI have been shown to have a lower QOL than the general population in a meta-analysis155. Out of functional impairment (the loss or abnormality of anatomical function), disability (a functional limitation for specific activities) and handicap (a disadvantage in acting in a certain role), handicap is most strongly associated with QOL in patients with SCI. Other studies have indicated that the severity and level of injury is significantly associated with QOL (that is, individuals with higher-level and more-severe injuries showed significantly lower QOL)156,157. However, conflicting results have been reported in other studies158,159. Other factors that are associated with QOL include advanced age and lower QOL160,161, and for both functional and psychological outcome, lengthier duration of SCI is associated with a more positive assessment of QOL160,162.
Social support, as indicated by marriage or cohabitation, and employment have a positive effect on QOL after SCI163,164. A higher level of education165 and the ability to walk without assistance161 were associated with higher QOL scores. SCI-related morbidities, including neuropathic pain, spasticity and bladder, bowel and sexual dysfunction were all associated with a lower QOL166. In general, carefully designed studies are required to give us a better understanding of how we can better prognosticate and inform long-term strategies to improve QOL for those with SCI.
Economic impact
The financial burden of SCI on patients, their families and society is substantial. Direct health care costs and living expenses vary substantially based on the geographical region and the age, survival and injury severity of individual patients. For example, in the United States, the lifetime cost for providing care to a patient 25 years of age with an ASIA Impairment Scale grade A injury is ∼$2.3 million for thoracic injuries, ∼$3.5 million for C5–C8 injuries and ∼$4.7 million for C1–C4 injuries over the course of the patient's lifetime. In addition, indirect costs (including lost wages and benefits) are estimated at ∼$72,000 per year2. Even small improvements in function, such as mobility and manual dexterity, can substantially reduce these costs, highlighting the economic importance of the ‘time is spine’ concept.
Outlook
Improving translation
Although numerous treatments have generated positive results in preclinical models of SCI, translation to patients has been challenging. Typically, preclinical studies use animal models with highly standardized injuries, treatment paradigms and assessment techniques in animals that are genotypically and phenotypically similar, which is in contrast to the heterogeneity of patients. As a result, an effective therapeutic approach from a single animal model might only be translatable to a subset of individuals within a clinical trial that often assesses a wide array of patients. This requires higher recruitment to sufficiently power the study and often necessitates controversial subgroup analyses167. To overcome this, one strategy is to embark on clinical trials only after a treatment has demonstrated efficacy in multiple animal models and species. Although this decreases the number of potentially translatable therapies, it might identify the highest yield treatments. Another strategy is to narrow the inclusion criteria of studies using clinical factors and biomarkers, which can provide insight into the underlying pathophysiology168. Together, these approaches might decrease variability in clinical trials (and recruitment requirements) while increasing the statistical power of the trial.
Common data elements
The SCI field needs high-quality large-scale data sets to better understand the heterogeneity between patients, as this affects their response to treatment and our ability to predict outcomes. Generating these data sets can be logistically challenging as patients present emergently and require complex care169, but several registries have been developed, including the North American Clinical Trials Network SCI Registry170, the International Spinal Cord Society SCI Data Sets171 and the National Spinal Cord Injury Statistical Center database172, among others. Large clinical trials have also contributed patient records, but data elements need to be standardized to harmonize data sets and to draw meaningful conclusions. Towards this goal, the National Institute of Neurological Disorders and Stroke (NINDS) within the US NIH has developed a set of common data elements for SCI. The 2014 common data elements are classified along a spectrum according to their use and validation in SCI and are grouped by field, including demographics, care, electrodiagnostics, functional, imaging, neurological, pain, QOL and psychological. Upcoming studies and registries should apply these elements to their data collection for the ultimate benefit of all patients.
Current clinical studies
The past several decades have seen a flurry of preclinical SCI research that has given rise to a host of promising therapeutic advances, each of which are in various stages of clinical development60,173. Pharmacological agents that are currently being investigated can broadly be classified as either neuroprotective or neuroregenerative (Table 1).
Neuroprotective treatments. Minocycline (a structural analogue of the antibiotic tetracycline) can induce neuroprotection in animal models of SCI, presumably through reducing oligodendrocyte apoptosis and by reducing local inflammation174,175. A phase II placebo-controlled, randomized study showed an improvement of 6 points in the ASIA motor score in 1 year after delivery of minocycline for 7 days compared with placebo, and only one adverse event — a transient increase in the levels of hepatic enzymes — was reported. A larger multicentre efficacy trial is currently ongoing (NCT01828203).
Riluzole (a sodium channel blocker) has improved neurobehavioural and pathological outcomes in animal models of SCI and is thought to prevent continuous activation of neuronal voltage-gated sodium channels, preventing cellular swelling and death, in addition to reducing excitotoxicity176. Data from a phase I trial showed an improvement in ASIA motor scores in patients with cervical level injuries, 90 days after riluzole treatment, compared with non-treated patients matched from a historical registry cohort177. Three patients had temporary borderline severe increases in the levels of liver enzymes, but no serious adverse events were attributed to the drug. Currently, a phase II/III multicentre, randomized trial (RISCIS) is enrolling patients and is supported by AOSpine (NCT01597518).
Basic fibroblast growth factor (bFGF; also known as fibroblast growth factor 2) is an important mediator of angiogenesis, has a key role as a morphogen in embryological development and is used in vitro to maintain pluripotency of many cells types, including neural stem cells178. In animal models, bFGF can promote neuroprotection against excitotoxicity and can reduce injury mediated by free radicals179,180. A structural analogue to bFGF (SUN13837) has been assessed in a phase I/II randomized trial with results pending.
Finally, the use of systemic hypothermia as a potential neuroprotective strategy is under clinical investigation in a phase II/III study: the ARCTIC trial. Hypothermia can decrease the basal metabolic rate of the CNS after injury and provides an anti-inflammatory effect181. Systemic intravascular cooling to 33 °C after acute hospital admission in patients with complete SCI is safe and associated with increased rates of ASIA Impairment Scale grade conversion compared with historical controls182.
Neuroregenerative treatments. The RHOA pathway can negatively affect axonal and neurite growth, and molecules that activate this pathway are upregulated following SCI183. A specific bacterially derived toxin, known as VX-210, can inhibit RHOA-mediated inhibition of axonal growth, leading to enhanced regeneration and improved behavioural outcomes in rodent models184. Cethrin (Alseres Pharmaceuticals), a recombinant version of VX-210, showed promise in preclinical studies and no serious drug-related adverse events were noted in a phase I/IIa dose-escalation study in patients with ASIA Impairment Scale grade A cervical and thoracic injuries185. Although this study was uncontrolled, ASIA motor score recovery at 12 months was superior to historical recovery rates185. A phase IIb/III study is under way (NCT02669849).
As previously mentioned, Nogo-A is found in CNS myelin and presumably has a role in preventing the formation of new functional connections post-SCI. Anti-Nogo-A antibodies have shown promise in promoting axonal regeneration in preclinical SCI studies186, and a phase I study has been completed, with a phase II placebo-controlled European trial under way187.
Biomaterials are under intense investigation, as they can be engineered to mimic the architecture of lost ECM in the spinal cord and can structurally support cell migration and axonal regrowth. A phase III trial in thoracic SCI, entitled INSPIRE, is under way in the United States, with a biodegradable Neuro-Spinal Scaffold (InVivo Therapeutics) to assess the safety and improvements in ASIA Impairment Scale grade, motor scores and sensory scores (NCT02138110).
Cellular transplantation. Transplantation of various cell types to repair the injured spinal cord is an exciting therapeutic concept188 and addresses the extensive loss of tissue caused by SCI that cannot be replaced by endogenous repair processes. In addition, transplanted cells can replace lost cells, modulate the injury environment and stimulate synergistic regenerative programmes173. Any specific cell type might have one or more of these actions, which remains an area of active investigation189.
The various cell types that have been assessed in preclinical studies include neural stem or precursor cells, oligodendrocyte precursor cells, olfactory ensheathing cells (OECs), Schwann cells and umbilical cord mesenchymal stem cells, among others173,190. Cell transplantation into the transected cord has been shown to promote the recovery of motor function, including coordinated walking191, paw use and climbing192, in addition to improved bladder function193 and phrenic nerve activity194 in animal models. Importantly, neural precursor cells and adult olfactory tissue are also effective when transplanted 1 month after SCI in rats, which is a time point that is considered to model stable, chronic SCI in humans.
Mechanistically, transplanted cells can improve regeneration by promoting axonal growth (observed with OECs), remyelinating denuded axons themselves (observed with Schwann cells and oligodendrocytes, among others) and supporting remyelination by endogenous oligodendrocytes195,196. In addition, factors that are secreted by transplanted cells can beneficially modulate the environment and promote axon regeneration197,198.
Several trials have tested the safety and preliminary efficacy of cell transplantation in patients with SCI. The first human trial confirmed the safety of transplantation of purified OECs into the spinal cord199. However, subsequently studies that transplanted mucosal tissue, as opposed to purified OECs, reported conflicting results200,201. Other trials have investigated other transplanted cells, including OECs and olfactory nerve fibroblasts, Schwann cells and a combination of OECs and Schwann cells202,203. A systematic review of the use of OECs in SCI supported the positive findings found in other trials204. More recently, data from a phase I trial reported motor and sensory improvements and no serious adverse events 1 year post-transplantation of autologous mucosal OECs and olfactory nerve fibroblasts into the spinal cords of patients with AISA Impairment Scale grade A injuries (n = 6)202. However, large sample sizes and long follow-up periods will be required to confirm safety and efficacy200.
Further efforts at cell transplantation include the transplantation of human embryonic stem cell-derived oligodendrocyte progenitor cells (such as the Geron trial), but this trial was discontinued for financial reasons. Fortunately, renewed funding has allowed Asterias Biotherapeutics, Inc. to restart the study of these cells as a phase I/IIa dose-escalation study, in which their product, AST-OPC1, is transplanted into the subacutely injured cervical spinal cord (NCT02302157). Other cell types that are under clinical investigation include human Schwann cells and umbilical cord blood mononuclear cells, among others. One phase I/II trial involving umbilical cord blood mononuclear cells found that the addition of intensive locomotor training to cell-based therapy can significantly enhance functional recovery in patients with chronic injuries205.
Biotechnology-led trials include the recently terminated testing of a human fetal neural stem cell product (HuCNS-SCs; StemCells, Inc.) and an ongoing trial by Neuralstem of transplanted NSI-566, which are stem cells derived from the human fetal spinal cord. Other possible therapies include adult autologous stem cells (RhinoCyte, Inc.), although this therapy is still at preclinical stages, and human glial-restricted progenitor product (Q-cells; Q Therapeutics). Numerous novel conventional drugs, such as anti-Nogo-A antibodies, are also currently being evaluated in clinical trials (Table 1).
Neuromodulation, robotics and future directions. Several neuromodulatory approaches, involving the focused delivery of electrical current to the CNS, are under study for the treatment of SCI. Specifically, spinal cord stimulation using surgically implanted electrodes in the epidural space over the conus medullaris, has improved functional and locomotion-related outcomes in patients with chronic SCI206. This trial is no longer active. In addition, although not under investigation in the clinic, preclinical studies have shown that stimulation of deep brain centres in the region of the mesencephalic locomotor region resulted in substantial improvement in functional deficit in rat SCI models207. Finally, neuroprosthetic brain–computer interfaces have successfully restored upper limb function in a paralysed patient; in this case, the device was implanted into the motor cortex of a patient with a complete cervical injury and stimulated specific hand and wrist muscle groups, which allowed functional control of motor output without transmission through the spinal cord208. The next steps are to reduce the movement-retraining process and to make this technology feasible for everyday use.
The use of robotics is also beginning to have a more substantive role in granting patients with SCI the ability regain functionality. In 2014, the US FDA approved the first robotic exoskeleton (ReWalk; ReWalk Robotics, Inc.) for use in patients with paraplegia, which fits around the legs and back of patients to facilitate sitting, standing and walking209,210. Other devices include Indego (Parker Hannifin Corporation), Ekso (Ekso Bionics), REX (Rex Bionics) and Hybrid Assistive Limb (Cyberdyne, Inc.)210. It is anticipated that, as technology improves, robotics will be used in conjunction with the discussed biological treatments to help optimize outcomes in the long term.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
How to cite this article
Fehlings, M. G. et al. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 3, 17018 (2017).
References
Noonan, V. K., Dvorak, M. F. & Fehlings, M. G. in Critical Care in Spinal Cord Injury (ed. Fehlings, M. G. ) 6–20 (Future Medicine Ltd, 2013).
[No authors listed.] Spinal cord injury facts and figures at a glance. J. Spinal Cord Med. 37, 117–118 (2014).
Singh, A., Tetreault, L., Kalsi-Ryan, S., Nouri, A. & Fehlings, M. G. Global prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol. 6, 309–331 (2014). A comprehensive overview of the global epidemiology of traumatic SCI divided by data available from geographical regions.
Cripps, R. A. et al. A global map for traumatic spinal cord injury epidemiology: towards a living data repository for injury prevention. Spinal Cord 49, 493–501 (2011).
Noonan, V. K. et al. Incidence and prevalence of spinal cord injury in Canada: a national perspective. Neuroepidemiology 38, 219–226 (2012).
New, P. W., Farry, A., Baxter, D. & Noonan, V. K. Prevalence of non-traumatic spinal cord injury in Victoria, Australia. Spinal Cord 51, 99–102 (2013).
Chen, Y., He, Y. & DeVivo, M. J. Changing demographics and injury profile of new traumatic spinal cord injuries in the United States, 1972–2014. Arch. Phys. Med. Rehabil. 97, 1610–1619 (2016). A cross-sectional analysis of longitudinal data that highlights key trends in the demographics of individuals with traumatic SCIs.
van den Berg, M. E., Castellote, J. M., Mahillo-Fernandez, I. & de Pedro-Cuesta, J. Incidence of spinal cord injury worldwide: a systematic review. Neuroepidemiology 34, 184–192 (2010).
Lenehan, B. et al. The epidemiology of traumatic spinal cord injury in British Columbia, Canada. Spine 37, 321–329 (2012).
Devivo, M. J. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord 50, 365–372 (2012).
DeVivo, M. J. & Chen, Y. Trends in new injuries, prevalent cases, and aging with spinal cord injury. Arch. Phys. Med. Rehabil. 92, 332–338 (2011).
Wu, J. C. et al. Effects of age, gender, and socio-economic status on the incidence of spinal cord injury: an assessment using the eleven-year comprehensive nationwide database of Taiwan. J. Neurotrauma 29, 889–897 (2012).
Wu, J. C. et al. Epidemiology of cervical spondylotic myelopathy and its risk of causing spinal cord injury: a national cohort study. Neurosurg. Focus 35, E10 (2013).
Krause, J., Sternberb, M., Lottes, S. & Maides, J. Mortality after spinal cord injury: an 11-year prospective study. Arch. Phys. Med. Rehabil. 78, 815–821 (1997).
LaPlaca, M. C., Simon, C. M., Prado, G. R. & Cullen, D. K. CNS injury biomechanics and experimental models. Prog. Brain Res. 161, 13–26 (2007).
Choo, A. M. et al. Contusion, dislocation, and distraction: primary hemorrhage and membrane permeability in distinct mechanisms of spinal cord injury. J. Neurosurg. Spine 6, 255–266 (2007).
Pineau, I. & Lacroix, S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 500, 267–285 (2007).
Schanne, F. A., Kane, A. B., Young, E. E. & Farber, J. L. Calcium dependence of toxic cell death: a final common pathway. Science 206, 700–702 (1979).
Kwon, B. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 4, 451–464 (2004).
Dizdaroglu, M., Jaruga, P., Birincioglu, M. & Rodriguez, H. Free radical-induced damage to DNA: mechanisms and measurement. Free Radic. Biol. Med. 32, 1102–1115 (2002).
Hausmann, O. N. Post-traumatic inflammation following spinal cord injury. Spinal Cord 41, 369–378 (2003).
Liu, M. et al. Necroptosis, a novel type of programmed cell death, contributes to early neural cells damage after spinal cord injury in adult mice. J. Spinal Cord Med. 38, 745–753 (2015).
Wang, Y. et al. Necroptosis inhibitor necrostatin-1 promotes cell protection and physiological function in traumatic spinal cord injury. Neuroscience 266, 91–101 (2014).
Li, S., Mealing, G. A., Morley, P. & Stys, P. K. Novel injury mechanism in anoxia and trauma of spinal cord white matter: glutamate release via reverse Na+-dependent glutamate transport. J. Neurosci. 19, RC16 (1999).
Li, S. & Stys, P. K. Mechanisms of ionotropic glutamate receptor-mediated excitotoxicity in isolated spinal cord white matter. J. Neurosci. 20, 1190–1198 (2000).
Norenberg, M. D., Smith, J. & Marcillo, A. The pathology of human spinal cord injury: defining the problems. J. Neurotrauma 21, 429–440 (2004).
Tator, C. H. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol. 5, 407–413 (1995).
Milhorat, T. H., Capocelli, A. L., Anzil, A. P., Kotzen, R. M. & Milhorat, R. H. Pathological basis of spinal cord cavitation in syringomyelia: analysis of 105 autopsy cases. J. Neurosurg. 82, 802–812 (1995).
McKeon, R. J., Schreiber, R. C., Rudge, J. S. & Silver, J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J. Neurosci. 11, 3398–3411 (1991).
Ahuja, C. S., Martin, A. R. & Fehlings, M. Recent advances in managing a spinal cord injury secondary to trauma. F1000Res. 5 (F1000 Faculty Rev), 1017 (2016).
Cregg, J. M. et al. Functional regeneration beyond the glial scar. Exp. Neurol. 253, 197–207 (2014).
Silver, J. & Miller, J. H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 5, 146–156 (2004). An in-depth review of the glial scar, ECM proteoglycans and the behaviour of regenerating neurons in the injured CNS microenvironment.
Wanner, I. B. et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J. Neurosci. 33, 12870–12886 (2013).
Anderson, M. A. et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature 532, 195–200 (2016).
Rolls, A., Shechter, R. & Schwartz, M. The bright side of the glial scar in CNS repair. Nat. Rev. Neurosci. 10, 235–241 (2009).
Filbin, M. T. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat. Rev. Neurosci. 4, 703–713 (2003).
Forgione, N. & Fehlings, M. G. Rho–ROCK inhibition in the treatment of spinal cord injury. World Neurosurg. 82, e535–e539 (2014).
Lee, J. K. et al. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron 66, 663–670 (2010).
Geoffroy, C. G. & Zheng, B. Myelin-associated inhibitors in axonal growth after CNS injury. Curr. Opin. Neurobiol. 27, 31–38 (2014).
Fehlings, M. G. & Tator, C. H. The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp. Neurol. 132, 220–228 (1995).
Tator, C. H. & Fehlings, M. G. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J. Neurosurg. 75, 15–26 (1991).
Kotter, M. R., Li, W. W., Zhao, C. & Franklin, R. J. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J. Neurosci. 26, 328–332 (2006).
Syed, Y. A. et al. Antibody-mediated neutralization of myelin-associated EphrinB3 accelerates CNS remyelination. Acta Neuropathol. 131, 281–298 (2016).
Kotter, M. R., Setzu, A., Sim, F. J., Van Rooijen, N. & Franklin, R. J. Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin-induced demyelination. Glia 35, 204–212 (2001).
Bieber, A. J., Warrington, A., Pease, L. R. & Rodriguez, M. Humoral autoimmunity as a mediator of CNS repair. Trends Neurosci. 24, S39–S44 (2001).
Jeffery, N. D. & Blakemore, W. F. Locomotor deficits induced by experimental spinal cord demyelination are abolished by spontaneous remyelination. Brain 120, 27–37 (1997).
Lynskey, J. V., Belanger, A. & Jung, R. Activity-dependent plasticity in spinal cord injury. J. Rehabil. Res. Dev. 45, 229–240 (2008).
Raineteau, O. & Schwab, M. E. Plasticity of motor systems after incomplete spinal cord injury. Nat. Rev. Neurosci. 2, 263–273 (2001).
Meletis, K. et al. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 6, e182 (2008).
Barnabe-Heider, F. et al. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 7, 470–482 (2010).
Mao, Y., Nguyen, T., Sutherland, T. & Gorrie, C. A. Endogenous neural progenitor cells in the repair of the injured spinal cord. Neural Regen. Res. 11, 1075–1076 (2016).
Bradbury, E. J. et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416, 636–640 (2002).
Li, G. et al. Mdivi-1 inhibits astrocyte activation and astroglial scar formation and enhances axonal regeneration after spinal cord injury in rats. Front. Cell. Neurosci. 10, 241 (2016).
Tator, C. H. Review of treatment trials in human spinal cord injury. Neurosurgery 59, 957–987 (2006).
Zhang, N., Fang, M., Chen, H., Gou, F. & Ding, M. Evaluation of spinal cord injury animal models. Neural Regen. Res. 9, 2008–2012 (2014).
Nout, Y. S. et al. Animal models of neurologic disorders: a nonhuman primate model of spinal cord injury. Neurotherapeutics 9, 380–392 (2012).
Kwon, B. K. et al. Large animal and primate models of spinal cord injury for the testing of novel therapies. Exp. Neurol. 269, 154–168 (2015).
Fisher, M. et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 40, 2244–2250 (2009).
Tator, C. H. Acute spinal cord injury: a review of recent studies of treatment and pathophysiology. Can. Med. Assoc. J. 107, 143–145 (1972).
Wilson, J. R., Forgione, N. & Fehlings, M. G. Emerging therapies for acute traumatic spinal cord injury. CMAJ 185, 485–492 (2013).
Guha, A., Tator, C. H. & Rochon, J. Spinal cord blood flow and systemic blood pressure after experimental spinal cord injury in rats. Stroke 20, 372–377 (1989).
Guha, A. B. & Tator, C. H. Acute cardiovascular effects of experimental spinal cord injury. J. Trauma 28, 481–490 (1988).
Schwab, J. M., Zhang, Y., Kopp, M. A., Brommer, B. & Popovich, P. G. The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury. Exp. Neurol. 258, 121–129 (2014).
Ko, H., Dittuno, J., Graziani, V. & Little, J. The pattern of reflex recovery during spinal shock. Spinal Cord 37, 402–409 (1999).
Ploumis, A., Yadlapalli, N., Fehlings, M. G., Kwon, B. K. & Vaccaro, A. R. A systematic review of the evidence supporting a role for vasopressor support in acute SCI. Spinal Cord 48, 356–362 (2010).
Lehmann, K. G., Lane, J. G., Piepmeier, J. M. & Batsford, W. P. Cardiovascular abnormalities accompanying acute spinal cord injury in humans: incidence, time course and severity. J. Am. Coll. Cardiol. 10, 46–52 (1987).
Guly, H. R., Bouamra, O., Lecky, F. E. & Trauma Audit and Research Network. The incidence of neurogenic shock in patients with isolated spinal cord injury in the emergency department. Resuscitation 76, 57–62 (2008).
Acheson, M., Livingston, R., Richardson, M. & Stimac, G. High-resolution CT scanning in the evaluation of cervical spine fractures: comparison with plain film examinations. AJR Am. J. Roentgenol. 148, 1179–1185 (1987).
Woodring, J. & Lee, C. Limitations of cervical radiography in the evaluation of acute cervical trauma. J. Trauma 34, 32–39 (1993).
Vaccaro, A. R. et al. AOSpine subaxial cervical spine injury classification system. Eur. Spine J. 25, 2173–2184 (2016).
Vaccaro, A. R. et al. AOSpine thoracolumbar spine injury classification system: fracture description, neurological status, and key modifiers. Spine 38, 2028–2037 (2013).
Lammertse, D. et al. Neuroimaging in traumatic spinal cord injury: an evidence-based review for clinical practice and research. J. Spinal Cord Med. 30, 205–214 (2007).
Miyanji, F., Furlan, J., Aarabi, B., Arnold, P. & Fehlings, M. Acute cervical traumatic spinal cord injury: MR imaging findings correlated with neurologic outcome — prospective study with 100 consecutive patients. Radiology 243, 820–827 (2007).
Vaccaro, A. R. et al. Magnetic resonance evaluation of the intervertebral disc, spinal ligaments, and spinal cord before and after closed traction reduction of cervical spine dislocations. Spine 24, 1210–1217 (1999).
Nakashima, H. et al. Posterior approach for cervical fracture-dislocations with traumatic disc herniation. Eur. Spine J. 20, 387–394 (2011).
Resnick, D. K. Updated guidelines for the management of acute cervical spine and spinal cord injury. Neurosurgery 72 (Suppl. 3), 1 (2013). Widely accepted guidelines by the AANS/CNS for the management of acute cervical SCIs.
Cadotte, D. W. & Fehlings, M. G. Will imaging biomarkers transform spinal cord injury trials? Lancet Neurol. 12, 843–844 (2013).
Curt, A. & Dietz, V. Electrophysiological recordings in patients with spinal cord injury: significance for predicting outcome. Spinal Cord 37, 157–165 (1999).
Curt, A., Van Hedel, H. J., Klaus, D., Dietz, V. & Group, E.-S. S. Recovery from a spinal cord injury: significance of compensation, neural plasticity, and repair. J. Neurotrauma 25, 677–685 (2008).
Kirshblum, S. C. et al. Reference for the 2011 revision of the International Standards for Neurological Classification of Spinal Cord Injury. J. Spinal Cord Med. 34, 547–554 (2011). The updated ISNCSCI for the assessment and reassessment of patients with traumatic SCI.
American Spinal Injury Association. ISNCSCI worksheet. ASIAhttp://asia-spinalinjury.org/information/downloads/ (2000).
Marino, R. J., Jones, L., Kirshblum, S., Tal, J. & Dasgupta, A. Reliability and repeatability of the motor and sensory examination of the international standards for neurological classification of spinal cord injury. J. Spinal Cord Med. 31, 166–170 (2008).
Savic, G., Bergstrom, E. M., Frankel, H. L., Jamous, M. A. & Jones, P. W. Inter-rater reliability of motor and sensory examinations performed according to American Spinal Injury Association standards. Spinal Cord 45, 444–451 (2007).
Sherwood, A. M., Dimitrijevic, M. R. & McKay, W. B. Evidence of subclinical brain influence in clinically complete spinal cord injury: discomplete SCI. J. Neurol. Sci. 110, 90–98 (1992).
Schneider, R. C., Cherry, G. & Pantek, H. The syndrome of acute central cervical spinal cord injury; with special reference to the mechanisms involved in hyperextension injuries of cervical spine. J. Neurosurg. 11, 546–577 (1954).
Fehlings, M. G. et al. The aging of the global population: the changing epidemiology of disease and spinal disorders. Neurosurgery 77, S1–S5 (2015).
McKinley, W., Santos, K., Meade, M. & Brooke, K. Incidence and outcomes of spinal cord injury clinical syndromes. J. Spinal Cord Med. 30, 215–224 (2007).
Burns, A. & Ditunno, J. Establishing prognosis and maximizing functional outcomes after spinal cord injury. Spine 26, S137–S145 (2001).
Kirshblum, S., Millis, S., McKinley, W. & Tulsky, D. Late neurologic recovery after traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 85, 1811–1817 (2004).
Fawcett, J. et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic trials. Spinal Cord 45, 190–205 (2007).
Marino, R., Dittuno, J., Donovan, W. & Maynard, F. Neurologic recovery after traumatic spinal cord injury: data from the model spinal cord injury systems. Arch. Phys. Med. Rehabil. 80, 1391–1396 (1999).
Waters, R., Yakura, J., Adkins, R. & Sie, I. Recovery following complete paraplegia. Arch. Phys. Med. Rehabil. 73, 784–789 (1992).
Coleman, W. & Geisler, F. Injury severity as primary predictor of outcome in acute spinal cord injury: retrospective results from a large multicenter clinical trial. Spine J. 4, 373–378 (2004).
Kay, E., Deutsch, A. & Wuermser, L. Predicting walking at discharge from inpatient rehabilitation after a traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 88, 745–750 (2007).
van Middendorp, J. J. et al. A clinical prediction rule for ambulation outcomes after traumatic spinal cord injury: a longitudinal cohort study. Lancet 377, 1004–1010 (2011). A clinical prediction rule for ambulation at 1 year post-SCI based on age, ASIA motor scores and sensory function in the subacute (within 15 days) injury period.
Wilson, J. R. et al. A clinical prediction model for long-term functional outcome after traumatic spinal cord injury based on acute clinical and imaging factors. J. Neurotrauma 29, 2263–2271 (2012).
Pavese, C. et al. Prediction of bladder outcomes after traumatic spinal cord injury: a longitudinal cohort study. PLoS Med. 13, e1002041 (2016).
Ryken, T. C. et al. The acute cardiopulmonary management of patients with cervical spinal cord injuries. Neurosurgery 72 (Suppl. 2), 84–92 (2013).
Braughler, J. & Hall, E. Effects of multi-dose methylprednisolone sodium succinate administration on injured cat spinal cord neurofilament degradation and energy metabolism. J. Neurosurg. 61, 290–295 (1984).
Hall, E. D. & Braughler, J. M. Glucocorticoid mechanisms in acute spinal cord injury: a review and therapeutic rationale. Surg. Neurol. 18, 320–327 (1982).
Hall, E. D. & Braughler, J. M. Effects of intravenous methylprednisolone on spinal cord lipid peroxidation and Na+ + K+)-ATPase activity. Dose–response analysis during 1st hour after contusion injury in the cat. J. Neurosurg. 57, 247–253 (1982).
Bracken, M. et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA 277, 1597–1604 (1997).
Bracken, M. B. et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N. Engl. J. Med. 322, 1405–1411 (1990).
Bracken, M. B. et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA 251, 45–52 (1984).
Bracken, M. B. Steroids for acute spinal cord injury. Cochrane Database Syst. Rev. 18, CD001046 (2012). A systematic review and meta-analysis of the best-available randomized trials that assess steroid therapy for acute traumatic SCI.
Eck, J. C., Nachtigall, D., Humphreys, S. C. & Hodges, S. D. Questionnaire survey of spine surgeons on the use of methylprednisolone for acute spinal cord injury. Spine 31, E250–E253 (2006).
Hurlbert, R. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J. Neurosurg. Spine 93, 1–7 (2000).
Hurlbert, R. J. et al. Pharmacological therapy for acute spinal cord injury. Neurosurgery 72 (Suppl. 2), 93–105 (2013).
Hadley, M. et al. Pharmacological therapy after acute cervical spinal cord injury. Neurosurgery 50, 563–572 (2002).
Fehlings, M. G., Wilson, J. R. & Cho, N. Methylprednisolone for the treatment of acute spinal cord injury: counterpoint. Neurosurgery 61 (Suppl. 1), 36–42 (2014).
Hurlbert, R. J. Methylprednisolone for the treatment of acute spinal cord injury: point. Neurosurgery 61 (Suppl. 1), 32–35 (2014).
Carlson, G. D. et al. Early time-dependent decompression for spinal cord injury: vascular mechanisms of recovery. J. Neurotrauma 14, 951–962 (1997).
Dimar, J., Glassman, S., Raque, G., Zhang, Y. & Shields, C. The influence of spinal canal narrowing and timing of decompression on neurologic recovery after spinal cord contusion in a rat model. Spine 24, 1623–1633 (1999).
Batchelor, P. E. et al. Meta-analysis of pre-clinical studies of early decompression in acute spinal cord injury: a battle of time and pressure. PLoS ONE 8, e72659 (2013).
Wilson, J. R. et al. Early versus late surgery for traumatic spinal cord injury: the results of a prospective Canadian cohort study. Spinal Cord 50, 840–843 (2012).
Bourassa-Moreau, E., Mac-Thiong, J. M., Feldman, D. E., Thompson, C. & Parent, S. Non-neurological outcomes after complete traumatic spinal cord injury: the impact of surgical timing. J. Neurotrauma 30, 1596–1601 (2013).
Grassner, L. et al. Early decompression (<8 h) after traumatic cervical spinal cord injury improves functional outcome as assessed by spinal cord independence measure after one year. J. Neurotrauma 33, 1658–1666 (2016).
Fehlings, M., Rabin, D., Sears, W., Cadotte, D. & Aarabi, B. Current practice in the timing of surgical intervention in spinal cord injury. Spine 35, 166–173 (2010).
Brodbelt, A. R. & Stoodley, M. A. Post-traumatic syringomyelia: a review. J. Clin. Neurosci. 10, 401–408 (2003).
Schurch, B., Wichmann, W. & Rossier, A. B. Post-traumatic syringomyelia (cystic myelopathy): a prospective study of 449 patients with spinal cord injury. J. Neurol. Neurosurg. Psychiatry 60, 61–67 (1996).
Alpert, S. W., Koval, K. J. & Zuckerman, J. D. Neuropathic arthropathy: review of current knowledge. J. Am. Acad. Orthop. Surg. 4, 100–108 (1996).
Aebli, N., Potzel, T. & Krebs, J. Characteristics and surgical management of neuropathic (Charcot) spinal arthropathy after spinal cord injury. Spine J. 14, 884–891 (2014).
Adams, M. M. & Hicks, A. L. Spasticity after spinal cord injury. Spinal Cord 43, 577–586 (2005).
Sezer, N., Akkus, S. & Ugurlu, F. G. Chronic complications of spinal cord injury. World J. Orthop. 6, 24–33 (2015).
Claydon, V. E., Steeves, J. D. & Krassioukov, A. Orthostatic hypotension following spinal cord injury: understanding clinical pathophysiology. Spinal Cord 44, 341–351 (2006).
Krassioukov, A., Eng, J. J., Warburton, D. E., Teasell, R. & Spinal Cord Injury Rehabilitation Evidence Research Team. A systematic review of the management of orthostatic hypotension after spinal cord injury. Arch. Phys. Med. Rehabil. 90, 876–885 (2009).
Krassioukov, A., Warburton, D. E., Teasell, R. & Eng, J. J. A systematic review of the management of autonomic dysreflexia after spinal cord injury. Arch. Phys. Med. Rehabil. 90, 682–695 (2009).
Blackmer, J. Rehabilitation medicine: 1. Autonomic dysreflexia. CMAJ 169, 931–935 (2003).
Linn, W. S., Adkins, R. H., Gong, H. Jr & Waters, R. L. Pulmonary function in chronic spinal cord injury: a cross-sectional survey of 222 southern California adult outpatients. Arch. Phys. Med. Rehabil. 81, 757–763 (2000).
Brown, R., DiMarco, A. F., Hoit, J. D. & Garshick, E. Respiratory dysfunction and management in spinal cord injury. Respir. Care 51, 853–868 (2006).
Winslow, C. & Rozovsky, J. Effect of spinal cord injury on the respiratory system. Am. J. Phys. Med. Rehabil. 82, 803–814 (2003).
DeVivo, M. J., Black, K. J. & Stover, S. L. Causes of death during the first 12 years after spinal cord injury. Arch. Phys. Med. Rehabil. 74, 248–254 (1993).
Brommer, B. et al. Spinal cord injury-induced immune deficiency syndrome enhances infection susceptibility dependent on lesion level. Brain 139, 692–707 (2016).
Ulndreaj, A., Chio, J. C., Ahuja, C. S. & Fehlings, M. G. Modulating the immune response in spinal cord injury. Expert Rev. Neurother. 16, 1127–1129 (2016).
Benevento, B. T. & Sipski, M. L. Neurogenic bladder, neurogenic bowel, and sexual dysfunction in people with spinal cord injury. Phys. Ther. 82, 601–612 (2002).
Taweel, W. A. & Seyam, R. Neurogenic bladder in spinal cord injury patients. Res. Rep. Urol. 7, 85–99 (2015).
Hess, M. J. & Hough, S. Impact of spinal cord injury on sexuality: broad-based clinical practice intervention and practical application. J. Spinal Cord Med. 35, 211–218 (2012).
Coggrave, M. J. & Norton, C. The need for manual evacuation and oral laxatives in the management of neurogenic bowel dysfunction after spinal cord injury: a randomized controlled trial of a stepwise protocol. Spinal Cord 48, 504–510 (2010).
Krassioukov, A., Eng, J. J., Claxton, G., Sakakibara, B. M. & Shum, S. Neurogenic bowel management after spinal cord injury: a systematic review of the evidence. Spinal Cord 48, 718–733 (2010).
Consortium for Spinal Cord Medicine Clinical Practice Guidelines. Pressure ulcer prevention and treatment following spinal cord injury: a clinical practice guideline for health-care professionals. J. Spinal Cord Med. 24, S40–S101 (2001).
van Kuijk, A. A., Geurts, A. C. & van Kuppevelt, H. J. Neurogenic heterotopic ossification in spinal cord injury. Spinal Cord 40, 313–326 (2002).
Rush, P. J. The rheumatic manifestations of traumatic spinal cord injury. Semin. Arthritis Rheum. 19, 77–89 (1989).
Cardenas, D. D. & Felix, E. R. Pain after spinal cord injury: a review of classification, treatment approaches, and treatment assessment. PM R 1, 1077–1090 (2009).
Gomara-Toldra, N., Sliwinski, M. & Dijkers, M. P. Physical therapy after spinal cord injury: a systematic review of treatments focused on participation. J. Spinal Cord Med. 37, 371–379 (2014).
Hwang, D. H. et al. Survival of neural stem cell grafts in the lesioned spinal cord is enhanced by a combination of treadmill locomotor training via insulin-like growth factor-1 signaling. J. Neurosci. 34, 12788–12800 (2014).
Dobkin, B. et al. Weight-supported treadmill versus over-ground training for walking after acute incomplete SCI. Neurology 66, 484–493 (2006).
Stiens, S. A., Kirshblum, S. C., Groah, S. L., McKinley, W. O. & Gittler, M. S. Spinal cord injury medicine. 4. Optimal participation in life after spinal cord injury: physical, psychosocial, and economic reintegration into the environment. Arch. Phys. Med. Rehabil. 83, S72–S81 (2002).
Ho, C. H. et al. Functional electrical stimulation and spinal cord injury. Phys. Med. Rehabil. Clin. N. Am. 25, 631–654 (2014).
Bhambhani, Y., Tuchak, C., Burnham, R., Jeon, J. & Maikala, R. Quadriceps muscle deoxygenation during functional electrical stimulation in adults with spinal cord injury. Spinal Cord 38, 630–638 (2000).
Kakebeeke, T. H. et al. Training and detraining of a tetraplegic subject: high-volume FES cycle training. Am. J. Phys. Med. Rehabil. 87, 56–64 (2008).
Ragnarsson, K. T. Functional electrical stimulation after spinal cord injury: current use, therapeutic effects and future directions. Spinal Cord 46, 255–274 (2008).
Tate, D. & Forchheimer, M. Review of cross-cultural issues related to quality of life after spinal cord injury. Top. Spinal Cord Inj. Rehabil. 20, 181–190 (2014).
Wilson, J. R., Hashimoto, R. E., Dettori, J. R. & Fehlings, M. G. Spinal cord injury and quality of life: a systematic review of outcome measures. Evid. Based Spine Care J. 2, 37–44 (2011).
Tulsky, D. S. et al. Overview of the Spinal Cord Injury–Quality of Life (SCI-QOL) measurement system. J. Spinal Cord Med. 38, 257–269 (2015).
Dijkers, M. Quality of life after spinal cord injury: a meta analysis of the effects of disablement components. Spinal Cord 35, 829–840 (1997). A meta-analysis of studies that assesses the relationship between QOL and disability, impairment and handicap.
Clayton, K. S. & Chubon, R. A. Factors associated with the quality of life of long-term spinal cord injured persons. Arch. Phys. Med. Rehabil. 75, 633–638 (1994).
Evans, R. L. et al. Quality of life after spinal cord injury: a literature critique and meta-analysis (1983–1992). J. Am. Paraplegia Soc. 17, 60–66 (1994).
Fuhrer, M. J., Rintala, D. H., Hart, K. A., Clearman, R. & Young, M. E. Relationship of life satisfaction to impairment, disability, and handicap among persons with spinal cord injury living in the community. Arch. Phys. Med. Rehabil. 73, 552–557 (1992).
Gerhart, K. A., Koziol-McLain, J., Lowenstein, S. R. & Whiteneck, G. G. Quality of life following spinal cord injury: knowledge and attitudes of emergency care providers. Ann. Emerg. Med. 23, 807–812 (1994).
Krause, J. S. & Crewe, N. M. Chronologic age, time since injury, and time of measurement: effect on adjustment after spinal cord injury. Arch. Phys. Med. Rehabil. 72, 91–100 (1991).
Jain, N. B., Sullivan, M., Kazis, L. E., Tun, C. G. & Garshick, E. Factors associated with health-related quality of life in chronic spinal cord injury. Am. J. Phys. Med. Rehabil. 86, 387–396 (2007). A cross-sectional study that investigates the health-related QOL instrument and identifies potentially modifiable factors related to QOL in SCI.
Brillhart, B. & Johnson, K. Motivation and the coping process of adults with disabilities: a qualitative study. Rehabil. Nurs. 22, 249–256 (1997).
Siosteen, A., Lundqvist, C., Blomstrand, C., Sullivan, L. & Sullivan, M. The quality of life of three functional spinal cord injury subgroups in a Swedish community. Paraplegia 28, 476–488 (1990).
DeVivo, M. J. & Richards, J. S. Community reintegration and quality of life following spinal cord injury. Paraplegia 30, 108–112 (1992).
Saadat, S. et al. Health-related quality of life among individuals with long-standing spinal cord injury: a comparative study of veterans and non-veterans. BMC Public Health 10, 6 (2010).
Westgren, N. & Levi, R. Quality of life and traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 79, 1433–1439 (1998).
Bracken, M. B. et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA 277, 1597–1604 (1997).
Kwon, B. K. et al. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J. Neurotrauma 27, 669–682 (2010).
Siddiqui, A. M., Khazaei, M. & Fehlings, M. G. Translating mechanisms of neuroprotection, regeneration, and repair to treatment of spinal cord injury. Prog. Brain Res. 218, 15–54 (2015).
Grossman, R. G., Toups, E. G., Frankowski, R. F., Burau, K. D. & Howley, S. North American Clinical Trials Network for the Treatment of Spinal Cord Injury: goals and progress. J. Neurosurg. Spine 17 (1 Suppl.), 6–10 (2012).
DeVivo, M. et al. International spinal cord injury core data set. Spinal Cord 44, 535–540 (2006).
DeVivo, M. J., Go, B. K. & Jackson, A. B. Overview of the national spinal cord injury statistical center database. J. Spinal Cord Med. 25, 335–338 (2002).
Ahuja, C. S. & Fehlings, M. Concise review: bridging the gap: novel neuroregenerative and neuroprotective strategies in spinal cord injury. Stem Cells Transl Med. 5, 914–924 (2016). A review of key neuroregenerative and neuroprotective interventions for traumatic SCI along the translational pipeline.
Wells, J., Hurlbert, R., Fehlings, M. & Yong, V. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain 126, 1628–1637 (2003).
Lee, S. M. et al. Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J. Neurotrauma 20, 1017–1027 (2003).
Wilson, J. R. & Fehlings, M. G. Riluzole for acute traumatic spinal cord injury: a promising neuroprotective treatment strategy. World Neurosurg. 81, 825–829 (2013).
Grossman, R. G. et al. A prospective, multicenter, phase I matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J. Neurotrauma 31, 239–255 (2014).
Santa-Olalla, J. & Covarrubias, L. Basic fibroblast growth factor promotes epidermal growth factor responsiveness and survival of mesencephalic neural precursor cells. J. Neurobiol. 40, 14–27 (1999).
Teng, Y. D., Mocchetti, I., Taveira-DaSilva, A. M., Gillis, R. A. & Wrathall, J. R. Basic fibroblast growth factor increases long-term survival of spinal motor neurons and improves respiratory function after experimental spinal cord injury. J. Neurosci. 19, 7037–7047 (1999).
Rabchevsky, A. G. et al. Basic fibroblast growth factor (bFGF) enhances functional recovery following severe spinal cord injury to the rat. Exp. Neurol. 164, 280–291 (2000).
Batchelor, P. E. et al. Hypothermia prior to decompression: buying time for treatment of acute spinal cord injury. J. Neurotrauma 27, 1357–1368 (2010).
Levi, A. D. et al. Clinical outcomes using modest intravascular hypothermia after acute cervical spinal cord injury. Neurosurgery 66, 670–677 (2010).
Dergham, P., Ellezam, B. & Essagian, C. Rho signalling pathway targeted to promote spinal cord repair. J. Neurosci. 22, 6570–6577 (2002).
Kwon, B. K., Sekhon, L. H. & Fehlings, M. G. Emerging repair, regeneration, and translational research advances for spinal cord injury. Spine 35, S263–S270 (2010).
Fehlings, M. G. et al. A phase I/IIa clinical trial of a recombinant Rho protein antagonist in acute spinal cord injury. J. Neurotrauma 28, 787–796 (2011).
Freund, P. et al. Anti-Nogo-A antibody treatment enhances sprouting of corticospinal axons rostral to a unilateral cervical spinal cord lesion in adult macaque monkey. J. Comp. Neurol. 502, 644–659 (2007).
Zorner, B. & Schwab, M. E. Anti-Nogo on the go: from animal models to a clinical trial. Ann. NY Acad. Sci. 1198 (Suppl. 1), E22–E34 (2010). A review of anti-Nogo therapy, which provides an example of the bench-to-bedside path for novel therapeutics.
Harrop, J. S. et al. Evaluation of clinical experience using cell-based therapies in patients with spinal cord injury: a systematic review. J. Neurosurg. Spine 17, 230–246 (2012).
Tetzlaff, W. et al. A systematic review of cellular transplantation therapies for spinal cord injury. J. Neurotrauma 28, 1611–1682 (2011).
Khazaei, M., Ahuja, C. S. & Fehlings, M. G. Induced pluripotent stem cells for traumatic spinal cord injury. Front. Cell Dev. Biol. 4, 152 (2017).
Ramon-Cueto, A., Cordero, M. I., Santos-Benito, F. F. & Avila, J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron 25, 425–435 (2000).
Keyvan-Fouladi, N., Raisman, G. & Li, Y. Functional repair of the corticospinal tract by delayed transplantation of olfactory ensheathing cells in adult rats. J. Neurosci. 23, 9428–9434 (2003).
Pascual, J. I., Gudino-Cabrera, G., Insausti, R. & Nieto-Sampedro, M. Spinal implants of olfactory ensheathing cells promote axon regeneration and bladder activity after bilateral lumbosacral dorsal rhizotomy in the adult rat. J. Urol. 167, 1522–1526 (2002).
Li, Y., Decherchi, P. & Raisman, G. Transplantation of olfactory ensheathing cells into spinal cord lesions restores breathing and climbing. J. Neurosci. 23, 727–731 (2003).
Boyd, J. G., Lee, J., Skihar, V., Doucette, R. & Kawaja, M. D. LacZ-expressing olfactory ensheathing cells do not associate with myelinated axons after implantation into the compressed spinal cord. Proc. Natl Acad. Sci. USA 101, 2162–2166 (2004).
Li, J. & Lepski, G. Cell transplantation for spinal cord injury: a systematic review. Biomed. Res. Int. 2013, 786475 (2013).
Pastrana, E. et al. Genes associated with adult axon regeneration promoted by olfactory ensheathing cells: a new role for matrix metalloproteinase 2. J. Neurosci. 26, 5347–5359 (2006).
Guo, J. S. et al. Cotransplant of neural stem cells and NT-3 gene modified Schwann cells promote the recovery of transected spinal cord injury. Spinal Cord 45, 15–24 (2007).
Mackay-Sim, A. et al. Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial. Brain 131, 2376–2386 (2008).
Dlouhy, B. J., Awe, O., Rao, R. C., Kirby, P. A. & Hitchon, P. W. Autograft-derived spinal cord mass following olfactory mucosal cell transplantation in a spinal cord injury patient: case report. J. Neurosurg. Spine 21, 618–622 (2014).
Chhabra, H. S. et al. Autologous olfactory [corrected] mucosal transplant in chronic spinal cord injury: an Indian pilot study. Spinal Cord 47, 887–895 (2009).
Tabakow, P. et al. Transplantation of autologous olfactory ensheathing cells in complete human spinal cord injury. Cell Transplant. 22, 1591–1612 (2013).
Hill, C. E., Moon, L. D., Wood, P. M. & Bunge, M. B. Labeled Schwann cell transplantation: cell loss, host Schwann cell replacement, and strategies to enhance survival. Glia 53, 338–343 (2006).
Li, L. et al. Effects of transplantation of olfactory ensheathing cells in chronic spinal cord injury: a systematic review and meta-analysis. Eur. Spine J. 24, 919–930 (2015). A meta-analysis that pools data from 1,193 patients receiving OEC transplants for chronic SCI.
Zhu, H. et al. Phase I–II clinical trial assessing safety and efficacy of umbilical cord blood mononuclear cell transplant therapy of chronic complete spinal cord injury. Cell Transplant. 25, 1925–1943(2016).
Harkema, S. et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377, 1938–1947 (2011).
Bachmann, L. C. et al. Deep brain stimulation of the midbrain locomotor region improves paretic hindlimb function after spinal cord injury in rats. Sci. Transl Med. 5, 208ra146 (2013).
Bouton, C. E. et al. Restoring cortical control of functional movement in a human with quadriplegia. Nature 533, 247–250 (2016).
Zeilig, G. et al. Safety and tolerance of the ReWalk exoskeleton suit for ambulation by people with complete spinal cord injury: a pilot study. J. Spinal Cord Med. 35, 96–101 (2012).
Miller, L. E., Zimmermann, A. K. & Herbert, W. G. Clinical effectiveness and safety of powered exoskeleton-assisted walking in patients with spinal cord injury: systematic review with meta-analysis. Med. Devices (Auckl.) 9, 455–466 (2016).
Akhtar, A. Z., Pippin, J. J. & Sandusky, C. B. Animal models in spinal cord injury: a review. Rev. Neurosci. 19 47–60 (2008).
Basso, D. M. Behavioral testing after spinal cord injury: congruities, complexities, and controversies. J. Neurotrauma 21, 395–404 (2004).
Kwon, B. K., Oxland, T. R. & Tetzlaff, W. Animal models used in spinal cord regeneration research. Spine 27, 1504–1510 (2002). An overview of pertinent animal models and injury paradigms to study spinal cord regeneration.
Calancie, B., Molano, M. R. & Broton, J. G. EMG for assessing the recovery of voluntary movement after acute spinal cord injury in man. Clin. Neurophysiol. 115, 1748–1759 (2004).
Curt, A. & Dietz, V. Nerve conduction study in cervical spinal cord injury: significance for hand function. NeuroRehabilitation 7, 165–173 (1996).
Jacobs, S. R., Yeaney, N. K., Herbison, G. J. & Ditunno, J. F. Jr . Future ambulation prognosis as predicted by somatosensory evoked potentials in motor complete and incomplete quadriplegia. Arch. Phys. Med. Rehabil. 76, 635–641 (1995).
Ogura, T. et al. Sympathetic skin response in patients with spinal cord injury. J. Orthop. Surg. (Hong Kong) 12, 35–39 (2004).
Bradbury, E. J. & McMahon, S. B. Spinal cord repair strategies: why do they work? Nat. Rev. Neurosci. 7, 644–653 (2006).
Takahashi, H. et al. Neuroprotective therapy using granulocyte colony-stimulating factor for acute spinal cord injury: a phase I/IIa clinical trial. Eur. Spine J. 21, 2580–2587 (2012).
Kamiya, K. et al. Neuroprotective therapy with granulocyte colony-stimulating factor in acute spinal cord injury: a comparison with high-dose methylprednisolone as a historical control. Eur. Spine J. 24, 963–967 (2015).
Lo, T. P. Jr et al. Systemic hypothermia improves histological and functional outcome after cervical spinal cord contusion in rats. J. Comp. Neurol. 514, 433–448 (2009).
Levi, A. D. et al. Clinical application of modest hypothermia after spinal cord injury. J. Neurotrauma 26, 407–415 (2009).
Author information
Authors and Affiliations
Contributions
Introduction (M.G.F., C.S.A., J.R.W., S.N., M.R.N.K. and C.D.); Epidemiology (S.N., C.S.A., J.R.W., M.R.N.K. and C.D.); Mechanisms/pathophysiology (M.G.F., C.S.A., J.R.W. and S.N.); Diagnosis, screening and prevention (C.S.A., J.R.W., S.N. and A.C.); Management (M.G.F., C.S.A., J.R.W., M.R.N.K., C.D. and A.C.); Quality of life (S.N., C.S.A., J.R.W., M.R.N.K. and C.D.); Outlook (C.S.A., J.R.W., S.N., M.R.N.K. and A.C.); Overview of Primer (M.G.F.). Co-first authors C.S.A. and J.R.W. contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
M.G.F. has consulting agreements with Pfizer, Zimmer Biomed and InVivo therapeutics. All other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Ahuja, C., Wilson, J., Nori, S. et al. Traumatic spinal cord injury. Nat Rev Dis Primers 3, 17018 (2017). https://doi.org/10.1038/nrdp.2017.18
Published:
DOI: https://doi.org/10.1038/nrdp.2017.18