Key Points
-
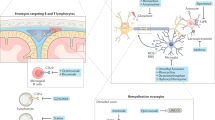
Progressive synaptic loss and dysfunction—also known as synaptopathy—occur early in multiple sclerosis (MS), and in experimental autoimmune encephalomyelitis (EAE), which is used to study MS in rodent models
-
Along with demyelination and axonal damage or transection, synaptopathy is a pathophysiological hallmark observed in MS and EAE; moreover, it is independent of axonal transection and demyelination
-
Synaptopathy has long-lasting effects that can be detrimental for motor and cognitive functions
-
In MS and EAE, neuroinflammation alters the balance between the GABAergic and glutamatergic systems in the brain and spinal cord
-
Proinflammatory cytokines released during acute MS attacks increase glutamate-mediated synaptic transmission and reduce γ-aminobutyric acid-mediated synaptic signalling, resulting in unbalanced synaptic hyperexcitation and possibly also to neurodegeneration
-
Targeting of mechanisms that stabilize, protect, repair or help regenerate synapses would enable clinical intervention at both early and late stages of the disease
Abstract
Multiple sclerosis (MS) has long been regarded as a chronic inflammatory disease of the white matter that leads to demyelination and eventually to neurodegeneration. In the past decade, several aspects of MS pathogenesis have been challenged, and degenerative changes of the grey matter, which are independent of demyelination, have become a topic of interest. CNS inflammation in MS and experimental autoimmune encephalomyelitis (EAE; a disease model used to study MS in rodents) causes a marked imbalance between GABAergic and glutamatergic transmission, and a loss of synapses, all of which leads to a diffuse 'synaptopathy'. Altered synaptic transmission can occur early in MS and EAE, independently of demyelination and axonal loss, and subsequently causes excitotoxic damage. Inflammation-driven synaptic abnormalities are emerging as a prominent pathogenic mechanism in MS—importantly, they are potentially reversible and, therefore, represent attractive therapeutic targets. In this Review, we focus on the connection between inflammation and synaptopathy in MS and EAE, which sheds light not only on the pathophysiology of MS but also on that of primary neurodegenerative disorders in which inflammatory processes contribute to disease progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Peterson, J. W., Bö, L., Mörk, S., Chang, A. & Trapp, B. D. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann. Neurol. 50, 389–400 (2001).
Compston, A. & Coles, A. Multiple sclerosis. Lancet 372, 1502–1517 (2008).
Ciccarelli, O. et al. Pathogenesis of multiple sclerosis: insights from molecular and metabolic imaging. Lancet Neurol. 13, 807–822 (2014).
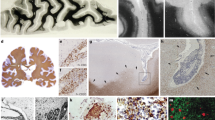
Geurts, J. J. G., Calabrese, M., Fisher, E. & Rudick, R. A. Measurement and clinical effect of grey matter pathology in multiple sclerosis. Lancet Neurol. 11, 1082–1092 (2012).
Bø, L., Vedeler, C. A., Nyland, H. I., Trapp, B. D. & Mørk, S. J. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J. Neuropathol. Exp. Neurol. 62, 723–732 (2003).
Kutzelnigg, A. et al. Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol. 17, 38–44 (2007).
Peterson, J. W., Bö, L., Mörk, S., Chang, A. & Trapp, B. D. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann. Neurol. 50, 389–400 (2001).
Wegner, C., Esiri, M. M., Chance, S. A., Palace, J. & Matthews, P. M. Neocortical neuronal, synaptic, and glial loss in multiple sclerosis. Neurology 67, 960–967 (2006).
Kooi, E.-J. et al. Cholinergic imbalance in the multiple sclerosis hippocampus. Acta Neuropathol. 122, 313–322 (2011).
De Stefano, N. et al. Evidence of early cortical atrophy in MS: relevance to white matter changes and disability. Neurology 60, 1157–1162 (2003).
Audoin, B. et al. Atrophy mainly affects the limbic system and the deep grey matter at the first stage of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 81, 690–695 (2010).
Calabrese, M. et al. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch. Neurol. 66, 1144–1150 (2009).
Tedeschi, G. et al. Brain atrophy and lesion load in a large population of patients with multiple sclerosis. Neurology 65, 280–285 (2005).
Sanfilipo, M. P., Benedict, R. H., Sharma, J., Weinstock-Guttman, B. & Bakshi, R. The relationship between whole brain volume and disability in multiple sclerosis: a comparison of normalized gray vs. white matter with misclassification correction. Neuroimage 26, 1068–1077 (2005).
Roosendaal, S. D. et al. Grey matter volume in a large cohort of MS patients: relation to MRI parameters and disability. Mult. Scler. 17, 1098–1106 (2011).
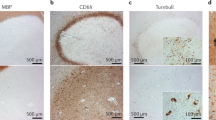
Dutta, R. et al. Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients. Ann. Neurol. 69, 445–454 (2011).
Calabrese, M. et al. Exploring the origins of grey matter damage in multiple sclerosis. Nat. Rev. Neurosci. 16, 147–158 (2015).
Mix, E., Meyer-Rienecker, H., Hartung, H. P. & Zettl, U. K. Animal models of multiple sclerosis—potentials and limitations. Prog. Neurobiol. 92, 386–404 (2010).
Friese, M. A., Schattling, B. & Fugger, L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat. Rev. Neurol. 10, 225–238 (2014).
Ellwardt, E. & Zipp, F. Molecular mechanisms linking neuroinflammation and neurodegeneration in MS. Exp. Neurol. 262, 8–17 (2014).
Michailidou, I. et al. Complement C1q-C3-associated synaptic changes in multiple sclerosis hippocampus. Ann. Neurol. 77, 1007–1026 (2015).
Vercellino, M. et al. Altered glutamate reuptake in relapsing-remitting and secondary progressive multiple sclerosis cortex: correlation with microglia infiltration, demyelination, and neuronal and synaptic damage. J. Neuropathol. Exp. Neurol. 66, 723–729 (2007).
Kornek, B. et al. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am. J. Pathol. 157, 267–276 (2000).
Tambalo, S. et al. Functional magnetic resonance imaging of rats with experimental autoimmune encephalomyelitis reveals brain cortex remodeling. J. Neurosci. 35, 10088–10100 (2015).
Zhu, B., Luo, L., Moore, G. R., Paty, D. W. & Cynader, M. S. Dendritic and synaptic pathology in experimental autoimmune encephalomyelitis. Am. J. Pathol. 162, 1639–1650 (2003).
Marques, K. B., Santos, L. M. & Oliveira, A. L. Spinal motoneuron synaptic plasticity during the course of an animal model of multiple sclerosis. Eur. J. Neurosci. 24, 3053–3062 (2006).
Freria, C. M., Zanon, R. G., Santos, L. M. & Oliveira, A. L. Major histocompatibility complex class I expression and glial reaction influence spinal motoneuron synaptic plasticity during the course of experimental autoimmune encephalomyelitis. J. Comp. Neurol. 518, 990–1007 (2010).
Kerschensteiner, M. et al. Remodeling of axonal connections contributes to recovery in an animal model of multiple sclerosis. J. Exp. Med. 200, 1027–1038 (2004).
Centonze, D. et al. Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J. Neurosci. 29, 3442–3452 (2009).
Ziehn, M. O., Avedisian, A. A., Tiwari-Woodruff, S. & Voskuhl, R. R. Hippocampal CA1 atrophy and synaptic loss during experimental autoimmune encephalomyelitis, EAE. Lab. Invest. 90, 774–786 (2010).
Ziehn, M. O. et al. Therapeutic testosterone administration preserves excitatory synaptic transmission in the hippocampus during autoimmune demyelinating disease. J. Neurosci. 32, 12312–12324 (2012).
Mori, F. et al. Interleukin-1β promotes long-term potentiation in patients with multiple sclerosis. Neuromolecular Med. 16, 38–51 (2013).
Mandolesi, G. et al. GABAergic signaling and connectivity on Purkinje cells are impaired in experimental autoimmune encephalomyelitis. Neurobiol. Dis. 46, 414–424 (2012).
Rossi, S. et al. Impaired striatal GABA transmission in experimental autoimmune encephalomyelitis. Brain Behav. Immun. 25, 947–956 (2011).
Falco, A., Pennucci, R., Brambilla, E. & De Curtis, I. Reduction in parvalbumin-positive interneurons and inhibitory input in the cortex of mice with experimental autoimmune encephalomyelitis. Exp. Brain Res. 232, 2439–2449 (2014).
Mandolesi, G. et al. Interleukin-1β alters glutamate transmission at purkinje cell synapses in a mouse model of multiple sclerosis. J. Neurosci. 33, 12105–12121 (2013).
Nisticò, R. et al. Inflammation subverts hippocampal synaptic plasticity in experimental multiple sclerosis. PLoS ONE 8, e54666 (2013).
Clements, R. J., McDonough, J. & Freeman, E. J. Distribution of parvalbumin and calretinin immunoreactive interneurons in motor cortex from multiple sclerosis post-mortem tissue. Exp. Brain Res. 187, 459–465 (2008).
Freeman, L. et al. The neuronal component of gray matter damage in multiple sclerosis: a [11C]flumazenil positron emission tomography study. Ann. Neurol. 78, 554–567 (2015).
Nicholas, R., Magliozzi, R., Campbell, G., Mahad, D. & Reynolds, R. Temporal lobe cortical pathology and inhibitory GABA interneuron cell loss are associated with seizures in multiple sclerosis. Mult. Scler. http://dx.doi.org/10.1177/1352458515579445.
Pitt, D., Werner, P. & Raine, C. S. Glutamate excitotoxicity in a model of multiple sclerosis. Nat. Med. 6, 67–70 (2000).
Stojanovic, I. R., Kostic, M. & Ljubisavljevic, S. The role of glutamate and its receptors in multiple sclerosis. J. Neural Transm. 121, 945–955 (2014).
Centonze, D. et al. The link between inflammation, synaptic transmission and neurodegeneration in multiple sclerosis. Cell Death Differ. 17, 1083–1091 (2010).
Azevedo, C. J. et al. In vivo evidence of glutamate toxicity in multiple sclerosis. Ann. Neurol. 76, 269–278 (2014).
Bolton, C. & Paul, C. Glutamate receptors in neuroinflammatory demyelinating disease. Mediators Inflamm. 2006, 93684 (2006).
Mehta, A., Prabhakar, M., Kumar, P., Deshmukh, R. & Sharma, P. L. Excitotoxicity: bridge to various triggers in neurodegenerative disorders. Eur. J. Pharmacol. 698, 6–18 (2013).
MacMillan, E. L. et al. Progressive multiple sclerosis exhibits decreasing glutamate and glutamine over two years. Mult. Scler. http://dx.doi.org/10.1177/1352458515586086.
Muhlert, N. et al. Memory in multiple sclerosis is linked to glutamate concentration in grey matter regions. J. Neurol. Neurosurg. Psychiatry 85, 834–840 (2014).
Qureshi, G. A. & Baig, M. S. Quantitation of free amino acids in biological samples by high-performance liquid chromatography. Application of the method in evaluating amino acid levels in cerebrospinal fluid and plasma of patients with multiple sclerosis. J. Chromatogr. 459, 237–244 (1988).
Honegger, C. G., Krenger, W. & Langemann, H. Changes in amino acid contents in the spinal cord and brainstem of rats with experimental autoimmune encephalomyelitis. J. Neurochem. 53, 423–427 (1989).
Musgrave, T. et al. The MAO inhibitor phenelzine improves functional outcomes in mice with experimental autoimmune encephalomyelitis (EAE). Brain Behav. Immun. 25, 1677–1688 (2011).
Kan, Q.-C., Zhang, S., Xu, Y.-M., Zhang, G.-X. & Zhu, L. Matrine regulates glutamate-related excitotoxic factors in experimental autoimmune encephalomyelitis. Neurosci. Lett. 560, 92–97 (2014).
Castegna, A. et al. Oxidative stress and reduced glutamine synthetase activity in the absence of inflammation in the cortex of mice with experimental allergic encephalomyelitis. Neuroscience 185, 97–105 (2011).
Werner, P., Pitt, D. & Raine, C. S. Multiple sclerosis: Altered glutamate homeostasis in lesions correlates with oligodendrocyre and axonal damage. Ann. Neurol. 50, 169–180 (2001).
Stover, J. F. et al. Neurotransmitters in cerebrospinal fluid reflect pathological activity. Eur. J. Clin. Invest. 27, 1038–1043 (1997).
Sarchielli, P., Greco, L., Floridi, A., Floridi, A. & Gallai, V. Excitatory amino acids and multiple sclerosis: evidence from cerebrospinal fluid. Arch. Neurol. 60, 1082–1088 (2003).
Srinivasan, R., Sailasuta, N., Hurd, R., Nelson, S. & Pelletier, D. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain 128, 1016–1025 (2005).
Vrenken, H. et al. MR spectroscopic evidence for glial increase but not for neuro-axonal damage in MS normal-appearing white matter. Magn. Reson. Med. 53, 256–266 (2005).
Baranzini, S. E. et al. Genetic variation influences glutamate concentrations in brains of patients with multiple sclerosis. Brain 133, 2603–2611 (2010).
Ganor, Y. & Levite, M. The neurotransmitter glutamate and human T cells: glutamate receptors and glutamate-induced direct and potent effects on normal human T cells, cancerous human leukemia and lymphoma T cells, and autoimmune human T cells. J. Neural Transm. 121, 983–1006 (2014).
Hardin-Pouzet, H. et al. Glutamate metabolism is down-regulated in astrocytes during experimental allergic encephalomyelitis. Glia 20, 79–85 (1997).
Olechowski, C. J. et al. A diminished response to formalin stimulation reveals a role for the glutamate transporters in the altered pain sensitivity of mice with experimental autoimmune encephalomyelitis (EAE). Pain 149, 565–572 (2010).
Ohgoh, M. Altered expression of glutamate transporters in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 125, 170–178 (2002).
Mitosek-Szewczyk, K., Sulkowski, G., Stelmasiak, Z. & Struz˙yn´ska, L. Expression of glutamate transporters GLT-1 and GLAST in different regions of rat brain during the course of experimental autoimmune encephalomyelitis. Neuroscience 155, 45–52 (2008).
Vallejo-Illarramendi, A., Domercq, M., Pérez-Cerdá, F., Ravid, R. & Matute, C. Increased expression and function of glutamate transporters in multiple sclerosis. Neurobiol. Dis. 21, 154–164 (2006).
Lieury, A. et al. Tissue remodeling in periplaque regions of multiple sclerosis spinal cord lesions. Glia 62, 1645–1658 (2014).
Newcombe, J. et al. Glutamate receptor expression in multiple sclerosis lesions. Brain Pathol. 18, 52–61 (2008).
Marte, A. et al. Alterations of glutamate release in the spinal cord of mice with experimental autoimmune encephalomyelitis. J. Neurochem. 115, 343–352 (2010).
Geurts, J. J. G. et al. Altered expression patterns of group I and II metabotropic glutamate receptors in multiple sclerosis. Brain 126, 1755–1766 (2003).
Fazio, F. et al. Switch in the expression of mGlu1 and mGlu5 metabotropic glutamate receptors in the cerebellum of mice developing experimental autoimmune encephalomyelitis and in autoptic cerebellar samples from patients with multiple sclerosis. Neuropharmacology 55, 491–499 (2008).
Han, M. H. et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature 451, 1076–1081 (2008).
Zhai, D. et al. Blocking GluR2-GAPDH ameliorates experimental autoimmune encephalomyelitis. Ann. Clin. Transl. Neurol. 2, 388–400 (2015).
Wheeler, E., Bolton, C., Mullins, J. & Paul, C. Identification of the NMDA receptor subtype involved in the development of disease during experimental autoimmune encephalomyelitis. pA2 [online], (2003).
Sulkowski, G., Dąbrowska-Bouta, B., Chalimoniuk, M. & Strużyńska, L. Effects of antagonists of glutamate receptors on pro-inflammatory cytokines in the brain cortex of rats subjected to experimental autoimmune encephalomyelitis. J. Neuroimmunol. 261, 67–76 (2013).
Groom, A. J., Smith, T. & Turski, L. Multiple sclerosis and glutamate. Ann. N. Y. Acad. Sci. 993, 229–275 (2003).
Starck, M., Albrecht, H., Pöllmann, W., Straube, A. & Dieterich, M. Drug therapy for acquired pendular nystagmus in multiple sclerosis. J. Neurol. 244, 9–16 (1997).
Plaut, G. S. Effectiveness of amantadine in reducing relapses in multiple sclerosis. J. R. Soc. Med. 80, 91–93 (1987).
Gilgun-Sherki, Y., Panet, H., Melamed, E. & Offen, D. Riluzole suppresses experimental autoimmune encephalomyelitis: Implications for the treatment of multiple sclerosis. Brain Res. 989, 196–204 (2003).
Shijie, J. et al. Blockade of glutamate release from microglia attenuates experimental autoimmune encephalomyelitis in mice. Tohoku J. Exp. Med. 217, 87–92 (2009).
Killestein, J., Kalkers, N. F. & Polman, C. H. Glutamate inhibition in MS: the neuroprotective properties of riluzole. J. Neurol. Sci. 233, 113–115 (2005).
Manyam, N. V., Katz, L., Hare, T. A., Gerber, J. C. & Grossman, M. H. Levels of gamma-aminobutyric acid in cerebrospinal fluid in various neurologic disorders. Arch. Neurol. 37, 352–355 (1980).
Gottesfeld, Z., Teitelbaum, D., Webb, C. & Arnon, R. Changes in the GABA system in experimental allergic encephalomyelitis-induced paralysis. J. Neurochem. 27, 695–699 (1976).
Achar, V. S., Welch, K. M., Chabi, E., Bartosh, K. & Meyer, J. S. Cerebrospinal fluid gamma-aminobutyric acid in neurologic disease. Neurology 26, 777–780 (1976).
Zepeda, A. T., Ortiz Nesme, F. J., Méndez-Franco, J., Otero-Siliceo, E. & Pérez de la Mora, M. Free-GABA levels in the cerebrospinal fluid of patients suffering from several neurological diseases Its potential use for the diagnosis of diseases which course with inflammation and tissular necrosis. Amino Acids 9, 207–216 (1995).
Demakova, E. V., Korobov, V. P. & Lemkina, L. M. Determination of gamma-aminobutyric acid concentration and activity of glutamate decarboxylase in blood serum of patients with multiple sclerosis [Russian]. Klin. Lab. Diagn. 4, 15–17 (2003).
Lock, C. et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 8, 500–508 (2002).
Massella, A. et al. Gender effect on neurodegeneration and myelin markers in an animal model for multiple sclerosis. BMC Neuroscience 13, 12 (2012).
Vanheel, A. et al. Identification of protein networks involved in the disease course of experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. PLoS ONE 7, e35544 (2012).
Wang, Y. et al. γ-Aminobutyric acid transporter 1 negatively regulates T cell-mediated immune responses and ameliorates autoimmune inflammation in the CNS. J. Immunol. 181, 8226–8236 (2008).
Paul, A. M. et al. GABA transport and neuroinflammation are coupled in multiple sclerosis: Regulation of the GABA transporter-2 by ganaxolone. Neuroscience 273, 24–38 (2014).
Tajouri, L. et al. Quantitative and qualitative changes in gene expression patterns characterize the activity of plaques in multiple sclerosis. Brain Res. Mol. Brain Res. 119, 170–183 (2003).
Bhat, R. et al. Inhibitory role for GABA in autoimmune inflammation. Proc. Natl Acad. Sci. USA 107, 2580–2585 (2010).
Jin, Z., Mendu, S. K. & Birnir, B. GABA is an effective immunomodulatory molecule. Amino Acids 45, 87–94 (2013).
Carmans, S. et al. Systemic treatment with the inhibitory neurotransmitter gamma-aminobutyric acid aggravates experimental autoimmune encephalomyelitis by affecting proinflammatory immune responses. J. Neuroimmunol. 255, 45–53 (2013).
Gilani, A. A., Dash, R. P., Jivrajani, M. N., Thakur, S. K. & Nivsarkar, M. Evaluation of GABAergic transmission modulation as a novel functional target for management of multiple sclerosis: Exploring inhibitory effect of GABA on glutamate-mediated excitotoxicity. Adv. Pharmacol. Sci. 2014, 632376 (2014).
Bibolini, M. J. et al. Inhibitory role of diazepam on autoimmune inflammation in rats with experimental autoimmune encephalomyelitis. Neuroscience 199, 421–428 (2011).
Rode, G., Maupas, E., Luaute, J., Courtois-Jacquin, S. & Boisson, D. Medical treatment of spasticity [French]. Neurochirurgie 49, 247–255 (2003).
Starck, M., Albrecht, H., Pöllmann, W., Dieterich, M. & Straube, A. Acquired pendular nystagmus in multiple sclerosis: an examiner-blind cross-over treatment study of memantine and gabapentin. J. Neurol. 257, 322–327 (2010).
Bandini, F., Castello, E., Mazzella, L., Mancardi, G. L. & Solaro, C. Gabapentin but not vigabatrin is effective in the treatment of acquired nystagmus in multiple sclerosis: how valid is the GABAergic hypothesis? J. Neurol. Neurosurg. Psychiatry 71, 107–110 (2001).
Cutter, N. C., Scott, D. D., Johnson, J. C. & Whiteneck, G. Gabapentin effect on spasticity in multiple sclerosis: a placebo-controlled, randomized trial. Arch. Phys. Med. Rehabil. 81, 164–169 (2000).
Grasselli, G. et al. Abnormal NMDA receptor function exacerbates experimental autoimmune encephalomyelitis. Br. J. Pharmacol. 168, 502–517 (2013).
Mallucci, G., Peruzzotti-Jametti, L., Bernstock, J. D. & Pluchino, S. The role of immune cells, glia and neurons in white and grey matter pathology in multiple sclerosis. Prog. Neurobiol. 127–128, 1–22 (2015).
Wrona, D. Neural-immune interactions: an integrative view of the bidirectional relationship between the brain and immune systems. J. Neuroimmunol. 172, 38–58 (2006).
Verderio, C. et al. Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann. Neurol. 72, 610–624 (2012).
Kettenmann, H., Kirchhoff, F. & Verkhratsky, A. Microglia: new roles for the synaptic stripper. Neuron 77, 10–18 (2013).
Vitkovic, L., Bockaert, J. & Jacque, C. “Inflammatory” cytokines: neuromodulators in normal brain? J. Neurochem. 74, 457–471 (2000).
Bauer, S., Kerr, B. J. & Patterson, P. H. The neuropoietic cytokine family in development, plasticity, disease and injury. Nat. Rev. Neurosci. 8, 221–232 (2007).
Schäfers, M. & Sorkin, L. Effect of cytokines on neuronal excitability. Neurosci. Lett. 437, 188–193 (2008).
Steinman, L. Inflammatory cytokines at the summits of pathological signal cascades in brain diseases. Sci. Signal. 6, pe3 (2013).
Rossi, S. et al. Interleukin-1β causes synaptic hyperexcitability in multiple sclerosis. Ann. Neurol. 71, 76–83 (2012).
Rossi, S. et al. Abnormal activity of the Na/Ca exchanger enhances glutamate transmission in experimental autoimmune encephalomyelitis. Brain Behav. Immun. 24, 1379–1385 (2010).
Haji, N. et al. TNF-α-mediated anxiety in a mouse model of multiple sclerosis. Exp. Neurol. 237, 296–303 (2012).
Weiss, S., Mori, F., Rossi, S. & Centonze, D. Disability in multiple sclerosis: when synaptic long-term potentiation fails. Neurosci. Biobehav. Rev. 43, 88–99 (2014).
Ziehn, M. O., Avedisian, A. A., Dervin, S. M., O'Dell, T. J. & Voskuhl, R. R. Estriol preserves synaptic transmission in the hippocampus during autoimmune demyelinating disease. Lab. Invest. 92, 1234–1245 (2012).
Musumeci, G. et al. Transient receptor potential vanilloid 1 channels modulate the synaptic effects of TNF-α and of IL-1β in experimental autoimmune encephalomyelitis. Neurobiol. Dis. 43, 669–677 (2011).
Rossini, P. M. et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr. Clin. Neurophysiol. 91, 79–92 (1994).
Conte, A. et al. Intracortical excitability in patients with relapsing-remitting and secondary progressive multiple sclerosis. J. Neurol. 256, 933–938 (2009).
Vucic, S. et al. Cortical dysfunction underlies disability in multiple sclerosis. Mult. Scler. 18, 425–432 (2012).
Schmierer, K., Irlbacher, K., Grosse, P., Röricht, S. & Meyer, B.-U. Correlates of disability in multiple sclerosis detected by transcranial magnetic stimulation. Neurology 59, 1218–1224 (2002).
Codecà, C. et al. Differential patterns of interhemispheric functional disconnection in mild and advanced multiple sclerosis. Mult. Scler. 16, 1308–1316 (2010).
Mori, F. et al. Short interval intracortical facilitation correlates with the degree of disability in multiple sclerosis. Brain Stimul. 6, 67–71 (2013).
Caramia, M. D. et al. 'Excitability changes of muscular responses to magnetic brain stimulation in patients with central motor disorders. Electroencephalogr. Clin. Neurophysiol. 81, 243–250 (1991).
Schwenkreis, P. et al. Influence of the N-methyl-D-aspartate antagonist memantine on human motor cortex excitability. Neurosci. Lett. 270, 137–140 (1999).
Ziemann, U., Lönnecker, S., Steinhoff, B. J. & Paulus, W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann. Neurol. 40, 367–378 (1996).
Rossi, S. et al. Inflammation inhibits GABA transmission in multiple sclerosis. Mult. Scler. 18, 1633–1635 (2012).
Rossi, S. et al. Tumor necrosis factor is elevated in progressive multiple sclerosis and causes excitotoxic neurodegeneration. Mult. Scler. 20, 304–312 (2014).
Morgen, K. et al. Training-dependent plasticity in patients with multiple sclerosis. Brain 127, 2506–2517 (2004).
Vosoughi, R. & Freedman, M. S. Therapy of MS. Clin. Neurol. Neurosurg. 112, 365–385 (2010).
Ransohoff, R. M., Hafler, D. A. & Lucchinetti, C. F. Multiple sclerosis—a quiet revolution. Nat. Rev. Neurol. 11, 134–142 (2015).
Franklin, R. J., ffrench-Constant, C., Edgar, J. M. & Smith, K. J. Neuroprotection and repair in multiple sclerosis. Nat. Rev. Neurol. 8, 624–634 (2012).
Arnold, D. L. et al. Effects of delayed-release dimethyl fumarate on MRI measures in the Phase 3 DEFINE study. J. Neurol. 261, 1794–1802 (2014).
Filippi, M. et al. Placebo-controlled trial of oral laquinimod in multiple sclerosis: MRI evidence of an effect on brain tissue damage. J. Neurol. Neurosurg. Psychiatry 85, 851–858 (2014).
Cohen, J. a. et al. Long-term (up to 4.5 years) treatment with fingolimod in multiple sclerosis: results from the extension of the randomised TRANSFORMS study. J. Neurol. Neurosurg. Psychiatry http://dx.doi.org/10.1136/jnnp-2015-310597.
Ontaneda, D., Fox, R. J. & Chataway, J. Clinical trials in progressive multiple sclerosis: lessons learned and future perspectives. Lancet Neurol. 14, 208–223 (2015).
Rossi, S. et al. Oral fingolimod rescues the functional deficits of synapses in experimental autoimmune encephalomyelitis. Br. J. Pharmacol. 165, 861–869 (2012).
Ruffini, F. et al. Laquinimod prevents inflammation-induced synaptic alterations occurring in experimental autoimmune encephalomyelitis. Mult. Scler. 19, 1084–1094 (2013).
Parodi, B. et al. Fumarates modulate microglia activation through a novel HCAR2 signaling pathway and rescue synaptic dysregulation in inflamed CNS. Acta Neuropathol. 130, 279–295 (2015).
Moore, S. et al. Therapeutic laquinimod treatment decreases inflammation, initiates axon remyelination, and improves motor deficit in a mouse model of multiple sclerosis. Brain Behav. 3, 664–682 (2013).
Brück, W. & Wegner, C. Insight into the mechanism of laquinimod action. J. Neurol. Sci. 306, 173–179 (2011).
Mishra, M. K. et al. Laquinimod reduces neuroaxonal injury through inhibiting microglial activation. Ann. Clin. Transl. Neurol. 1, 409–422 (2014).
Wilms, H. et al. Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1β, TNF-α and IL-6 in an in-vitro model of brain inflammation. J. Neuroinflammation 7, 30 (2010).
Noda, H., Takeuchi, H., Mizuno, T. & Suzumura, A. Fingolimod phosphate promotes the neuroprotective effects of microglia. J. Neuroimmunol. 256, 13–18 (2013).
Di Menna, L. et al. Fingolimod protects cultured cortical neurons against excitotoxic death. Pharmacol. Res. 67, 1–9 (2013).
Landi, D. et al. Oral fingolimod reduces glutamate-mediated intracortical excitability in relapsing-remitting multiple sclerosis. Clin. Neurophysiol. 126, 165–169 (2015).
Glass, C. K., Saijo, K., Winner, B., Marchetto, M. C. & Gage, H. Mechanisms underlying inflammation in neurodegeneration. Cell 140, 918–934 (2010).
Lovera, J. F. et al. Memantine for cognitive impairment in multiple sclerosis: a randomized placebo-controlled trial. Mult. Scler. 16, 715–723 (2010).
Smith, T., Groom, A., Zhu, B. & Turski, L. Autoimmune encephalomyelitis ameliorated by AMPA antagonists. Nat. Med. 6, 62–66 (2000).
Bolton, C. & Paul, C. MK-801 limits neurovascular dysfunction during experimental allergic encephalomyelitis. J. Pharmacol. Exp. Ther. 282, 397–402 (1997).
Paul, C. & Bolton, C. Modulation of blood-brain barrier dysfunction and neurological deficits during acute experimental allergic encephalomyelitis by the N-methyl-D-aspartate receptor antagonist memantine. J. Pharmacol. Exp. Ther. 302, 50–57 (2002).
Wallström, E. et al. Memantine abrogates neurological deficits, but not CNS inflammation, in Lewis rat experimental autoimmune encephalomyelitis. J. Neurol. Sci. 137, 89–96 (1996).
Sulkowski, G., Dąbrowska-Bouta, B., Salińska, E. & Strużyńska, L. Modulation of glutamate transport and receptor binding by glutamate receptor antagonists in EAE rat brain. PLoS ONE 9, e113954 (2014).
Sulkowski, G., Dąbrowska-Bouta, B., & Strużyńska, L. Modulation of neurological deficits and expression of glutamate receptors during experimental autoimmune encephalomyelitis after treatment with selected antagonists of glutamate receptors. Biomed. Res. Int. 2013, 186068 (2013).
Kanwar, J. R., Kanwar, R. K. & Krissansen, G. W. Simultaneous neuroprotection and blockade of inflammation reverses autoimmune encephalomyelitis. Brain 127, 1313–1331 (2004).
Kanwar, J. R. Anti-inflammatory immunotherapy for multiple sclerosis/experimental autoimmune encephalomyelitis (EAE) disease. Curr. Med. Chem. 12, 2947–2962 (2005).
Besong, G. et al. Activation of group III metabotropic glutamate receptors inhibits the production of RANTES in glial cell cultures. J. Neurosci. 22, 5403–5411 (2002).
Solaro, C., Uccelli, M. M., Guglieri, P., Uccelli, A. & Mancardi, G. L. Gabapentin is effective in treating nocturnal painful spasms in multiple sclerosis. Mult. Scler. 6, 192–193 (2000).
Ørsnes, G., Crone, C., Krarup, C., Petersen, N. & Nielsen, J. The effect of baclofen on the transmission in spinal pathways in spastic multiple sclerosis patients. Clin. Neurophysiol. 111, 1372–1379 (2000).
Nielsen, J. F., Anderson, J. B. & Sinkjaer, T. Baclofen increases the soleus stretch reflex threshold in the early swing phase during walking in spastic multiple sclerosis patients. Mult. Scler. 6, 105–114 (2000).
Nielsen, J. F. & Sinkjær, T. Peripheral and central effect of baclofen on ankle joint stiffness in multiple sclerosis. Muscle Nerve 23, 98–105 (2000).
Acknowledgements
This work was supported by the Italian National Ministero della Salute (Progetto Giovani Ricercatori) to G.M. (No. GR-2011-02347036) and A.M. (No. GR-2011-02351422) and by Fondazione Italiana Sclerosi Multipla (No. FISM 2010/S/2) to D.C.
Author information
Authors and Affiliations
Contributions
D.C., G.M., A.G., A.M., D.F., F.D.V., S.B. and H.S. researched data for the article. D.C. and G.M. wrote the article. D.C., G.M., A.G., A.M. and G.A.M. provided substantial contributions to discussion of content. All authors participated in reviewing and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
D.C. is an Advisory Board member for Bayer Schering, Merck-Serono and Teva, and has received honoraria for speaking or consultation fees from Almirall, Bayer Schering, Biogen Idec, Genzyme, GW Pharmaceuticals, Merck Serono, Novartis, Sanofi-Aventis and Teva. He is also the principal investigator in clinical trials for Bayer Schering, Biogen Idec, Novartis, Merck Serono, Sanofi-Aventis and Teva. The other authors declare no competing interests.
Supplementary information
Supplementary Table 1
Evidence for glutamatergic system dysfunction in MS (DOCX 34 kb)
Supplementary Table 2
Evidence for glutamatergic system dysfunction in EAE (DOCX 33 kb)
Supplementary Table 3
Evidence for GABAergic system dysfunction in MS and EAE (DOCX 29 kb)
Supplementary Table 4
Inflammation-dependent synaptic dysfunction in EAE models of MS (DOCX 61 kb)
Rights and permissions
About this article
Cite this article
Mandolesi, G., Gentile, A., Musella, A. et al. Synaptopathy connects inflammation and neurodegeneration in multiple sclerosis. Nat Rev Neurol 11, 711–724 (2015). https://doi.org/10.1038/nrneurol.2015.222
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2015.222
This article is cited by
-
Nogo-A antibody delivery through the olfactory mucosa mitigates experimental autoimmune encephalomyelitis in the mouse CNS
Cell Death Discovery (2023)
-
Glia Connect Inflammation and Neurodegeneration in Multiple Sclerosis
Neuroscience Bulletin (2023)
-
Cerebellar connectome alterations and associated genetic signatures in multiple sclerosis and neuromyelitis optica spectrum disorder
Journal of Translational Medicine (2023)
-
ISGylation is induced in neurons by demyelination driving ISG15-dependent microglial activation
Journal of Neuroinflammation (2022)
-
Genes associated with grey matter volume reduction in multiple sclerosis
Journal of Neurology (2022)