Key Points
-
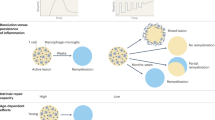
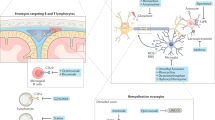
CNS remyelination serves to limit and repair the damage in demyelinating diseases such as multiple sclerosis (MS)
-
Several intrinsic molecular pathways that execute endogenous remyelination have been identified and are potential therapeutic targets
-
The first clinical proof-of-concept trials to enhance remyelination in MS have been conducted in the past few years
-
The optimal clinical and paraclinical outcome measures for the assessment of remyelination are not known, but neurophysiological measures, MRI, myelin-targeted PET radiotracers, and optical coherence tomography all are possible adjuncts to clinical outcomes in proof-of-concept studies
-
The timing of remyelination therapy is a crucial issue
-
Future MS therapy is likely to involve a combination of immunomodulatory and regenerative treatments
Abstract
Remyelination in the CNS is the natural process of damage repair in demyelinating diseases such as multiple sclerosis (MS). However, remyelination becomes inadequate in many people with MS, which results in axonal degeneration and clinical disability. Enhancement of remyelination is a logical therapeutic goal; nevertheless, all currently licensed therapies for MS are immunomodulatory and do not support remyelination directly. Several molecular pathways have been identified as potential therapeutic targets to induce remyelination, and some of these have now been assessed in proof-of-concept clinical trials. However, trial design faces several obstacles: optimal clinical or paraclinical outcome measures to assess remyelination remain ill-defined, and identification of the ideal timing of therapy is also a crucial issue. In addition, realistic expectations are needed concerning the probable benefits of such therapies. Nevertheless, approaches that enhance remyelination are likely to be protective for axons and so could prevent long-term neurodegeneration. Future MS treatment paradigms, therefore, are likely to comprise a combinatorial approach that involves both immunomodulatory and regenerative treatments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hemmer, B., Kerschensteiner, M. & Korn, T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol. 14, 406–419 (2015).
Dendrou, C. A., Fugger, L. & Friese, M. A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 15, 545–558 (2015).
Stangel, M. & Hartung, H. P. Remyelinating strategies for the treatment of multiple sclerosis. Prog. Neurobiol. 68, 361–376 (2002).
Franklin, R. J. & ffrench-Constant, C. Remyelination in the CNS: from biology to therapy. Nat. Rev. Neurosci. 9, 839–855 (2008).
Gaesser, J. M. & Fyffe-Maricich, S. L. Intracellular signaling pathway regulation of myelination and remyelination in the CNS. Exp. Neurol. 283, 501–511 (2016).
Kremer, D., Gottle, P., Hartung, H. P. & Kury, P. Pushing forward: remyelination as the new frontier in CNS diseases. Trends Neurosci. 39, 246–263 (2016).
Ford, M. C. et al. Tuning of Ranvier node and internode properties in myelinated axons to adjust action potential timing. Nat. Commun. 6, 8073 (2015).
Nave, K. A. & Werner, H. B. Myelination of the nervous system: mechanisms and functions. Annu. Rev. Cell Dev. Biol. 30, 503–533 (2014).
Simons, M., Misgeld, T. & Kerschensteiner, M. A unified cell biological perspective on axon-myelin injury. J. Cell Biol. 206, 335–345 (2014).
Cerina, M. et al. The quality of cortical network function recovery depends on localization and degree of axonal demyelination. Brain Behav. Immun. 59, 103–117 (2017).
Prineas, J. W. & Connell, F. Remyelination in multiple sclerosis. Ann. Neurol. 5, 22–31 (1979).
Prineas, J. W., Kwon, E. E., Cho, E. S. & Sharer, L. R. Continual breakdown and regeneration of myelin in progressive multiple sclerosis plaques. Ann. NY Acad. Sci. 436, 11–32 (1984).
Esiri, M. M. & Morris, C. S. Immunocytochemical study of macrophages and microglial cells and extracellular matrix components in human CNS disease 2. Non-neoplastic diseases. J. Neurol. Sci. 101, 59–72 (1991).
Stidworthy, M. F., Genoud, S., Suter, U., Mantei, N. & Franklin, R. J. Quantifying the early stages of remyelination following cuprizone-induced demyelination. Brain Pathol. 13, 329–339 (2003).
Patrikios, P. et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain 129, 3165–3172 (2006).
Albert, M., Antel, J., Bruck, W. & Stadelmann, C. Extensive cortical remyelination in patients with chronic multiple sclerosis. Brain Pathol. 17, 129–138 (2007).
Goldschmidt, T., Antel, J., Konig, F. B., Brück, W. & Kuhlmann, T. Remyelination capacity of the MS brain decreases with disease chronicity. Neurology 72, 1914–1921 (2009).
Marques, S. et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352, 1326–1329 (2016).
Xing, Y. L. et al. Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. J. Neurosci. 34, 14128–14146 (2014).
Samanta, J. et al. Inhibition of Gli1 mobilizes endogenous neural stem cells for remyelination. Nature 526, 448–452 (2015).
Patani, R., Balaratnam, M., Vora, A. & Reynolds, R. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol. Appl. Neurobiol. 33, 277–287 (2007).
Kuhlmann, T. et al. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 131, 1749–1758 (2008).
Mensch, S. et al. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat. Neurosci. 18, 628–630 (2015).
Chen, J. T., Collins, D. L., Atkins, H. L., Freedman, M. S. & Arnold, D. L. Magnetization transfer ratio evolution with demyelination and remyelination in multiple sclerosis lesions. Ann. Neurol. 63, 254–262 (2008).
Brown, R. A., Narayanan, S. & Arnold, D. L. Imaging of repeated episodes of demyelination and remyelination in multiple sclerosis. Neuroimage Clin. 6, 20–25 (2014).
Powers, B. E. et al. Remyelination reporter reveals prolonged refinement of spontaneously regenerated myelin. Proc. Natl Acad. Sci. USA 110, 4075–4080 (2013).
Boyd, A., Zhang, H. & Williams, A. Insufficient OPC migration into demyelinated lesions is a cause of poor remyelination in MS and mouse models. Acta Neuropathol. 125, 841–859 (2013).
Wolswijk, G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J. Neurosci. 18, 601–609 (1998).
Chang, A., Tourtellotte, W. W., Rudick, R. & Trapp, B. D. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N. Engl. J. Med. 346, 165–173 (2002).
Bechler, M. E., Byrne, L. & Ffrench-Constant, C. CNS myelin sheath lengths are an intrinsic property of oligodendrocytes. Curr. Biol. 25, 2411–2416 (2015).
Lee, S., Chong, S. Y., Tuck, S. J., Corey, J. M. & Chan, J. R. A rapid and reproducible assay for modeling myelination by oligodendrocytes using engineered nanofibers. Nat. Protoc. 8, 771–782 (2013).
Lee, S. et al. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat. Methods 9, 917–922 (2012).
Wake, H. et al. Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nat. Commun. 6, 7844 (2015).
Hines, J. H., Ravanelli, A. M., Schwindt, R., Scott, E. K. & Appel, B. Neuronal activity biases axon selection for myelination in vivo. Nat. Neurosci. 18, 683–689 (2015).
Gibson, E. M. et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304 (2014).
Gautier, H. O. et al. Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Nat. Commun. 6, 8518 (2015).
Lundgaard, I. et al. Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol. 11, e1001743 (2013).
Jarjour, A. A. & Kennedy, T. E. Oligodendrocyte precursors on the move: mechanisms directing migration. Neuroscientist 10, 99–105 (2004).
Gutowski, N. J., Newcombe, J. & Cuzner, M. L. Tenascin-R and C in multiple sclerosis lesions: relevance to extracellular matrix remodelling. Neuropathol. Appl. Neurobiol. 25, 207–214 (1999).
Sobel, R. A. & Mitchell, M. E. Fibronectin in multiple sclerosis lesions. Am. J. Pathol. 135, 161–168 (1989).
Sobel, R. A., Chen, M., Maeda, A. & Hinojoza, J. R. Vitronectin and integrin vitronectin receptor localization in multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 54, 202–213 (1995).
Stoffels, J. M. et al. Fibronectin aggregation in multiple sclerosis lesions impairs remyelination. Brain 136, 116–131 (2013).
Tepavcevic, V. et al. Early netrin-1 expression impairs central nervous system remyelination. Ann. Neurol. 76, 252–268 (2014).
Williams, A. et al. Semaphorin 3A and 3F: key players in myelin repair in multiple sclerosis? Brain 130, 2554–2565 (2007).
Li, H. & Richardson, W. D. Evolution of the CNS myelin gene regulatory program. Brain Res. 1641, 111–121 (2016).
Fancy, S. P. et al. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 23, 1571–1585 (2009).
Fancy, S. P. et al. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat. Neurosci. 14, 1009–1016 (2011).
Huang, J. K. et al. Retinoid X receptor γ signaling accelerates CNS remyelination. Nat. Neurosci. 14, 45–53 (2011).
Mi, S. et al. LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis. Nat. Med. 13, 1228–1233 (2007).
Mi, S. et al. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat. Neurosci. 8, 745–751 (2005).
Deshmukh, V. A. et al. A regenerative approach to the treatment of multiple sclerosis. Nature 502, 327–332 (2013).
Najm, F. J. et al. Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature 522, 216–220 (2015).
Mei, F. et al. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat. Med. 20, 954–960 (2014).
Baer, A. S. et al. Myelin-mediated inhibition of oligodendrocyte precursor differentiation can be overcome by pharmacological modulation of Fyn-RhoA and protein kinase C signalling. Brain 132, 465–481 (2009).
Ruckh, J. M. et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 10, 96–103 (2012).
Lau, L. W., Cua, R., Keough, M. B., Haylock-Jacobs, S. & Yong, V. W. Pathophysiology of the brain extracellular matrix: a new target for remyelination. Nat. Rev. Neurosci. 14, 722–729 (2013).
Back, S. A. et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat. Med. 11, 966–972 (2005).
Mishra, M. K. & Yong, V. W. Myeloid cells — targets of medication in multiple sclerosis. Nat. Rev. Neurol. 12, 539–551 (2016).
Rothhammer, V. & Quintana, F. J. Control of autoimmune CNS inflammation by astrocytes. Semin. Immunopathol. 37, 625–638 (2015).
Correale, J. & Farez, M. F. The role of astrocytes in multiple sclerosis progression. Front. Neurol. 6, 180 (2015).
Skripuletz, T. et al. Astrocytes regulate myelin clearance through recruitment of microglia during cuprizone-induced demyelination. Brain 136, 147–167 (2013).
Skripuletz, T. et al. Pivotal role of choline metabolites in remyelination. Brain 138, 398–413 (2015).
Miron, V. E. Microglia-driven regulation of oligodendrocyte lineage cells, myelination, and remyelination. J. Leukoc. Biol. 101, 1103–1108 (2017).
Li, J. et al. Astrocytes in oligodendrocyte lineage development and white matter pathology. Front. Cell Neurosci. 10, 119 (2016).
Xiao, J. et al. Mesenchymal stem cells and induced pluripotent stem cells as therapies for multiple sclerosis. Int. J. Mol. Sci. 16, 9283–9302 (2015).
Salinas Tejedor, L. et al. Mesenchymal stem cells do not exert direct beneficial effects on CNS remyelination in the absence of the peripheral immune system. Brain Behav. Immun. 50, 155–165 (2015).
Bjartmar, C., Kinkel, R. P., Kidd, G., Rudick, R. A. & Trapp, B. D. Axonal loss in normal-appearing white matter in a patient with acute MS. Neurology 57, 1248–1252 (2001).
Brown, R. A., Narayanan, S., Banwell, B., Arnold, D. L. & Canadian Pediatric Demyelinating Disease Network. Magnetization transfer ratio recovery in new lesions decreases during adolescence in pediatric-onset multiple sclerosis patients. Neuroimage Clin. 6, 237–242 (2014).
Gudi, V., Gingele, S., Skripuletz, T. & Stangel, M. Glial response during cuprizone-induced de- and remyelination in the CNS: lessons learned. Front. Cell Neurosci. 8, 73 (2014).
Thorpe, J. W. et al. Serial gadolinium-enhanced MRI of the brain and spinal cord in early relapsing-remitting multiple sclerosis. Neurology 46, 373–378 (1996).
Green, A. et al. Positive phase II double-blind randomized placebo-controlled crossover trial of clemastine [abstract]. American Academy of Neurology 68th Annual Meeting https://www.aan.com/PressRoom/Home/GetDigitalAsset/12049 (2016).
Stangel, M., Boegner, F., Klatt, C. H., Hofmeister, C. & Seyfert, S. A placebo-controlled pilot trial to study the remyelinating potential of intravenous immunoglobulins in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 68, 89–92 (2000).
Brusa, A., Jones, S. J. & Plant, G. T. Long-term remyelination after optic neuritis: a 2-year visual evoked potential and psychophysical serial study. Brain 124, 468–479 (2001).
Balcer, L. J. et al. Low-contrast acuity measures visual improvement in phase 3 trial of natalizumab in relapsing MS. J. Neurol. Sci. 318, 119–124 (2012).
Waxman, S. G. Axonal conduction and injury in multiple sclerosis: the role of sodium channels. Nat. Rev. Neurosci. 7, 932–941 (2006).
Sarnthein, J., Andersson, M., Zimmermann, M. B. & Zumsteg, D. High test-retest reliability of checkerboard reversal visual evoked potentials (VEP) over 8 months. Clin. Neurophysiol. 120, 1835–1840 (2009).
Costello, F. E., Klistorner, A. & Kardon, R. Optical coherence tomography in the diagnosis and management of optic neuritis and multiple sclerosis. Ophthalm. Surg. Lasers Imag. 42, S28–S40 (2011).
Talman, L. S. et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann. Neurol. 67, 749–760 (2010).
Mallik, S., Samson, R. S., Wheeler-Kingshott, C. A. & Miller, D. H. Imaging outcomes for trials of remyelination in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 85, 1396–1404 (2014).
Matthews, P. M. & Datta, G. Positron-emission tomography molecular imaging of glia and myelin in drug discovery for multiple sclerosis. Expert Opin. Drug Discov. 10, 557–570 (2015).
Schmierer, K., Parkes, H. G. & So, P. W. Direct visualization of remyelination in multiple sclerosis using T2-weighted high-field MRI. Neurology 72, 472 (2009).
Alonso-Ortiz, E., Levesque, I. R. & Pike, G. B. MRI-based myelin water imaging: a technical review. Magn. Reson. Med. 73, 70–81 (2015).
Laule, C. et al. Myelin water imaging in multiple sclerosis: quantitative correlations with histopathology. Mult. Scler. 12, 747–753 (2006).
Vavasour, I. M. et al. Longitudinal changes in myelin water fraction in two MS patients with active disease. J. Neurol. Sci. 276, 49–53 (2009).
Vargas, W. S. et al. Measuring longitudinal myelin water fraction in new multiple sclerosis lesions. Neuroimage Clin. 9, 369–375 (2015).
Duval, T. et al. g-Ratio weighted imaging of the human spinal cord in vivo. Neuroimage 145, 11–23 (2017).
Alexander, A. L., Lee, J. E., Lazar, M. & Field, A. S. Diffusion tensor imaging of the brain. NeuroTherapeutics 4, 316–329 (2007).
Fox, R. J. et al. A validation study of multicenter diffusion tensor imaging: reliability of fractional anisotropy and diffusivity values. AJNR Am. J. Neuroradiol. 33, 695–700 (2012).
Chiang, C. W. et al. Quantifying white matter tract diffusion parameters in the presence of increased extra-fiber cellularity and vasogenic edema. Neuroimage 101, 310–319 (2014).
Fujiyoshi, K. et al. Application of q-space diffusion MRI for the visualization of white matter. J. Neurosci. 36, 2796–2808 (2016).
Giacomini, P. S. et al. Measuring demyelination and remyelination in acute multiple sclerosis lesion voxels. Arch. Neurol. 66, 375–381 (2009).
Schmierer, K., Scaravilli, F., Altmann, D. R., Barker, G. J. & Miller, D. H. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann. Neurol. 56, 407–415 (2004).
Altmann, D. R. et al. Sample sizes for lesion magnetisation transfer ratio outcomes in remyelination trials for multiple sclerosis. Mult. Scler. Relat. Disord. 3, 237–243 (2014).
Schwartzbach, C. J. et al. Lesion remyelinating activity of GSK239512 versus placebo in patients with relapsing–remitting multiple sclerosis: a randomised, single-blind, phase II study. J. Neurol. 264, 304–315 (2016).
Levesque, I. R. et al. Quantitative magnetization transfer and myelin water imaging of the evolution of acute multiple sclerosis lesions. Magn. Reson. Med. 63, 633–640 (2010).
Veronese, M. et al. Quantification of [11C]PIB PET for imaging myelin in the human brain: a test-retest reproducibility study in high-resolution research tomography. J. Cereb. Blood Flow Metab. 35, 1771–1782 (2015).
Bodini, B. et al. Dynamic imaging of individual remyelination profiles in multiple sclerosis. Ann. Neurol. 79, 726–738 (2016).
Tiwari, A. D. et al. Design, synthesis, and evaluation of fluorinated radioligands for myelin imaging. J. Med. Chem. 59, 3705–3718 (2016).
Maggi, P. et al. The formation of inflammatory demyelinated lesions in cerebral white matter. Ann. Neurol. 76, 594–608 (2014).
Datta, G. et al. 11C-PBR28 or 18F-PBR111 detect white matter inflammatory heterogeneity in multiple sclerosis. J. Nucl. Med. 58, 1477–1482 (2017).
van Waesberghe, J. H. et al. Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann. Neurol. 46, 747–754 (1999).
Huang, S. Y. et al. Characterization of axonal disease in patients with multiple sclerosis using high-gradient-diffusion MR imaging. Radiology 280, 244–251 (2016).
Lucchinetti, C. et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann. Neurol. 47, 707–717 (2000).
Gupta, N. et al. Neural stem cell engraftment and myelination in the human brain. Sci. Transl. Med. 4, 155ra137 (2012).
Jokubaitis, V. G. et al. Predictors of disability worsening in clinically isolated syndrome. Ann. Clin. Transl. Neurol. 2, 479–491 (2015).
Brown, R. A., Narayanan, S. & Arnold, D. L. Segmentation of magnetization transfer ratio lesions for longitudinal analysis of demyelination and remyelination in multiple sclerosis. Neuroimage 66, 103–109 (2013).
Cadavid, D. et al. Safety and efficacy of opicinumab in acute optic neuritis (RENEW): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 16, 189–199 (2017).
Cadavid, D. et al. Efficacy analysis of opicinumab in relapsing multiple sclerosis: the Phase 2b SYNERGY trial [abstract]. ECTRIMS https://Onlinelibrary.Ectrims-Congress.eu/Ectrims/2016/32nd/147038/Diego.Cadavid.Efficacy.Analysis.of.Opicinumab.in.Relaps.Multiple.Sclerosis.Html?f=m1 (2016).
Suhs, K. W. et al. A randomized, double-blind, phase 2 study of erythropoietin in optic neuritis. Ann. Neurol. 72, 199–210 (2012).
John, G. R. et al. Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat. Med. 8, 1115–1121 (2002).
Stidworthy, M. F. et al. Notch1 and Jagged1 are expressed after CNS demyelination, but are not a major rate-determining factor during remyelination. Brain 127, 1928–1941 (2004).
Zhang, Y. et al. Notch1 signaling plays a role in regulating precursor differentiation during CNS remyelination. Proc. Natl Acad. Sci. USA 106, 19162–19167 (2009).
Furusho, M., Kaga, Y., Ishii, A., Hebert, J. M. & Bansal, R. Fibroblast growth factor signaling is required for the generation of oligodendrocyte progenitors from the embryonic forebrain. J. Neurosci. 31, 5055–5066 (2011).
Lindner, M. et al. Fibroblast growth factor signalling in multiple sclerosis: inhibition of myelination and induction of pro-inflammatory environment by FGF9. Brain 138, 1875–1893 (2015).
Mohan, H. et al. Transcript profiling of different types of multiple sclerosis lesions yields FGF1 as a promoter of remyelination. Acta Neuropathol. Commun. 2, 168 (2014).
Mi, S. et al. Promotion of central nervous system remyelination by induced differentiation of oligodendrocyte precursor cells. Ann. Neurol. 65, 304–315 (2009).
Mi, S. et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat. Neurosci. 7, 221–228 (2004).
Hu, Q. D. et al. F3/contactin acts as a functional ligand for Notch during oligodendrocyte maturation. Cell 115, 163–175 (2003).
Nakahara, J., Kanekura, K., Nawa, M., Aiso, S. & Suzuki, N. Abnormal expression of TIP30 and arrested nucleocytoplasmic transport within oligodendrocyte precursor cells in multiple sclerosis. J. Clin. Invest. 119, 169–181 (2009).
Kotter, M. R., Li, W. W., Zhao, C. & Franklin, R. J. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J. Neurosci. 26, 328–332 (2006).
Syed, Y. A. et al. Antibody-mediated neutralization of myelin-associated EphrinB3 accelerates CNS remyelination. Acta Neuropathol. 131, 281–298 (2016).
Natrajan, M. S. et al. Retinoid X receptor activation reverses age-related deficiencies in myelin debris phagocytosis and remyelination. Brain 138, 3581–3597 (2015).
Miron, V. E. et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 16, 1211–1218 (2013).
Mei, F. et al. Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. eLife 5, e18246 (2016).
Charles, P. et al. Negative regulation of central nervous system myelination by polysialyated-neural cell adhesion molecule. Proc. Natl Acad. Sci. USA 97, 7585–7590 (2000).
Charles, P. et al. Re-expression of PSA-NCAM by demyelinated axons: an inhibitor of remyelination in multiple sclerosis? Brain 125, 1972–1979 (2002).
Yuen, T. J. et al. Identification of endothelin 2 as an inflammatory factor that promotes central nervous system remyelination. Brain 136, 1035–1047 (2013).
Hammond, T. R. et al. Astrocyte-derived endothelin-1 inhibits remyelination through Notch activation. Neuron 81, 588–602 (2014).
Al Nimer, F. et al. Lipocalin-2 is increased in progressive multiple sclerosis and inhibits remyelination. Neurol. Neuroimmunol. Neuroinflamm. 3, e191 (2016).
Bai, L. et al. Hepatocyte growth factor mediates mesenchymal stem cell-induced recovery in multiple sclerosis models. Nat. Neurosci. 15, 862–870 (2012).
Sloane, J. A. et al. Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc. Natl Acad. Sci. USA 107, 11555–11560 (2010).
Piaton, G. et al. Class 3 semaphorins influence oligodendrocyte precursor recruitment and remyelination in adult central nervous system. Brain 134, 1156–1167 (2011).
Spassky, N. et al. Directional guidance of oligodendroglial migration by class 3 semaphorins and netrin-1. J. Neurosci. 22, 5992–6004 (2002).
Clemente, D., Ortega, M. C., Arenzana, F. J. & de Castro, F. FGF-2 and Anosmin-1 are selectively expressed in different types of multiple sclerosis lesions. J. Neurosci. 31, 14899–14909 (2011).
Acknowledgements
This study was supported by grants to T.K. from the German Research Foundation (SFB-TR128-B7; Ku1477/6-1) and the Interdisciplinary Clinical Research Center, Münster (IZKF; KuT3/012/15). P.M.M. is in receipt of personal and research support from the Edmond J. Safra Foundation and Lily Safra, the Imperial College National Institute for Health Research (NIHR) Biomedical Research Centre, the NIHR Senior Investigator Programme, the UK Dementia Research Institute, the UK Medical Research Council, Biogen and the Engineering and Physics Science Research Council. M.S. is supported by Niedersachsen Research Network on Neuroinfectiology (N-RENNT) of the Ministry of Science and Culture of Lower Saxony.
Author information
Authors and Affiliations
Contributions
All authors performed literature searches, selected important literature to be cited, wrote various sections of the manuscript, and revised the entire manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- G-ratio
-
The ratio of the inner axonal diameter to the total outer diameter, an index of relative myelin thickness
- Uhthoff's phenomenon
-
Heat-associated worsening of neurological symptoms caused by inhibition of nerve conduction following demyelination.
- Shadow plaques
-
Multiple sclerosis lesions in which the total lesion area is remyelinated.
- Heterochronic parabiosis
-
Surgical joining of two animals of different ages to generate a shared circulation.
- Optical coherence tomography
-
An optical imaging method that provides detailed information regarding the microstructure of thin layers of tissue (for example, the retina) based on backscatter of reflected light.
- Diffusion-weighted MRI
-
An MRI method in which the contrast-reflecting tissue microstructure is determined based on intrinsic local differences in the rate or extent of diffusion of water.
- b-value
-
A scanner parameter conveying the relative strength and timing of the diffusion-sensitising gradients for diffusion-weighted MRI; stronger diffusion effects are imaged with higher b-values.
- Q-ball imaging
-
A form of diffusion MRI developed by Dr David Tuch of Harvard University, USA, which enables multiple white matter fibre orientations to beresolved even within a single voxel.
- Magnetization transfer imaging
-
An imaging method based on the intrinsic contrast provided by differences in the extent of transfer of magnetisation from a macromolecular bound water pool (for example, myelin water) to the bulk water when radiofrequency energy is applied to the bound water pool.
Rights and permissions
About this article
Cite this article
Stangel, M., Kuhlmann, T., Matthews, P. et al. Achievements and obstacles of remyelinating therapies in multiple sclerosis. Nat Rev Neurol 13, 742–754 (2017). https://doi.org/10.1038/nrneurol.2017.139
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2017.139
This article is cited by
-
Conversion of Astrocyte Cell Lines to Oligodendrocyte Progenitor Cells Using Small Molecules and Transplantation to Animal Model of Multiple Sclerosis
Journal of Molecular Neuroscience (2024)
-
Icariin ameliorates the cuprizone-induced demyelination associated with antioxidation and anti-inflammation
Inflammopharmacology (2024)
-
Inflammation in multiple sclerosis: consequences for remyelination and disease progression
Nature Reviews Neurology (2023)
-
Thinking outside the box: non-canonical targets in multiple sclerosis
Nature Reviews Drug Discovery (2022)
-
Chronic oligodendrocyte injury in central nervous system pathologies
Communications Biology (2022)