Abstract
Background
Newborns with hypoxic–ischemic encephalopathy (HIE) may exhibit abnormalities on placental histology. In this phase II clinical trial ancillary study, we hypothesized that placental abnormalities correlate with MRI brain injury and with response to treatment.
Methods
Fifty newborns with moderate/severe encephalopathy who received hypothermia were enrolled in a double-blind, placebo-controlled trial of erythropoietin for HIE. A study pathologist reviewed all available clinical pathology reports to determine the presence of chronic abnormalities and acute chorioamnionitis. Neonatal brain MRIs were scored using a validated HIE scoring system.
Results
Placental abnormalities in 19 of the 35 (54%) patients with available pathology reports included chronic changes (N = 13), acute chorioamnionitis (N = 9), or both (N = 3). MRI subcortical brain injury was less common in infants with a placental abnormality (26 vs. 69%, P = 0.02). Erythropoietin treatment was associated with a lower global brain injury score (median 2.0 vs. 11.5, P = 0.003) and lower rate of subcortical brain injury (33 vs. 90%, P = 0.01) among patients with no chronic placental abnormality but not in patients whose placentas harbored a chronic abnormality.
Conclusion
Erythropoietin treatment was associated with less brain injury only in patients whose placentas exhibited no chronic histologic changes. Placentas may provide clues to treatment response in HIE.
Similar content being viewed by others
Introduction
Hypoxic–ischemic encephalopathy (HIE), an important cause of neonatal encephalopathy (NE) and neonatal brain injury, occurs in 1–3 per 1000 term births1,2 and is responsible for 23% of neonatal deaths worldwide.3 HIE is typically diagnosed in infants born with low Apgar scores, cord blood or early neonatal acidemia, need for prolonged resuscitation, and moderate-to-severe clinical signs of encephalopathy.4,5 Survivors of HIE are at risk for brain injury and life-long neurodevelopmental disabilities, such as cerebral palsy and cognitive impairment.4 The complex causal pathways underlying HIE are poorly understood.6 Potential contributing factors include antenatal conditions such as advanced maternal age, infertility treatment, intrauterine growth restriction, and maternal hypertension, as well as intrapartum complications, such as maternal infection and sentinel events (e.g., placental abruption, uterine rupture).7,8,9,10,11,12,13,14 A better understanding of the timing and pathophysiology underlying HIE could inform new strategies to prevent this condition and could help tailor specific therapies to subsets of infants with HIE.
Placental gas exchange and nutrient delivery are vital to the health of the fetus both during fetal development and during the labor and delivery process. It is reasonable then to suspect that placental abnormalities may confer susceptibility to HIE and hypoxic–ischemic brain injury. Several placental findings have indeed been reported in term infants diagnosed with HIE, NE, or cerebral palsy, including histologic chorioamnionitis15,16,17, placental vascular abnormalities or infarction15,18,19, and chronic villitis of unknown etiology.20 A recent study of NE was the first to examine placentas from both case and control deliveries and found that placental fetal vascular malperfusion imparted a 3-fold increased risk of encephalopathy (odds ratio (OR) 3.2, 95% confidence interval (CI) 1.5–6.6).19
Previous treatment trials of HIE have rarely included placental data. Although hypothermia, the only proven therapy for HIE, may be less effective in patients with placental findings of chorioamnionitis16 or chronic villitis17, there are no available data from hypothermia clinical trials to either support or refute this hypothesis.
In the Neonatal Erythropoietin and Therapeutic Hypothermia Outcomes (NEATO) phase II randomized, double-blind placebo-controlled trial, we found that high doses of erythropoietin (Epo) in addition to therapeutic hypothermia resulted in less brain injury when compared to treatment with hypothermia alone.5 We now report the placental findings in patients enrolled in the NEATO trial. We examine whether placental abnormalities were associated with brain injury or 12-month neurodevelopment and whether response to Epo treatment differed between those with and without placental abnormalities.
Subjects and methods
Subjects
Detailed methods of the NEATO trial (NCT 01913340) have been previously published.5 Briefly, 50 newborns with moderate/severe HIE were randomized at 7 tertiary care centers to receive either Epo 1000 U/kg intravenously or an equal volume of normal saline on days 1, 2, 3, 5, and 7. All subjects met 4 inclusion criteria: (1) ≥36 weeks gestation; (2) perinatal depression defined as at least 1 of the following: 10-min Apgar score <5; need for on-going resuscitation at 10 min; or cord or neonatal acidosis (i.e., pH <7.00 or base deficit ≥15) within 1 h of birth; (3) moderate/severe encephalopathy based on Sarnat examination; and (4) hypothermia initiated by 6 h of age. Exclusion criteria included congenital anomalies, suspected genetic syndrome, birth weight <1800 g, head circumference <5% for gestational age, and redirection of care being considered because of moribund condition.
Demographics and complications of labor and delivery were ascertained prospectively at the time of neonatal hospitalization. Clinical chorioamnionitis was defined as a diagnosis of chorioamnionitis assigned by a treating physician based on clinical symptoms alone. A sentinel event was defined as the presence of a prolapsed cord, placental abruption, uterine rupture, or shoulder dystocia. Baseline plasma Epo levels were measured in blood samples taken within 24 h of age, prior to administration of study drug. Birth weight and head circumference <10% and >90% were determined based on population norms for gestational age and sex.
Placental analysis
Placental pathologic examination occurred at the birth hospitals as part of routine clinical care. All placental pathology reports were retrieved and reviewed centrally by a study pathologist (R.W.R.) who was blinded to treatment allocation and to brain magnetic resonance imaging (MRI) findings. Chronic placental abnormalities were defined as any of the following: (1) maternal vascular malperfusion (i.e., villous infarct, accelerated villous maturation, increased syncytial knots); (2) fetal vascular malperfusion (i.e., focal avascular villi and/or thrombus in fetal vessel); or (3) villitis of unknown etiology. Acute placental abnormalities were defined by the presence of a neutrophilic maternal and/or fetal inflammatory response in the chorionic plate, membranes, or major fetal vessels (i.e., histologic chorioamnionitis). Placental weight and fetoplacental ratio were categorized as low (<10%) or high (>90%) based on population normative tables.21
Neuroimaging
Patients received a brain MRI between 4 and 7 days of age as part of routine clinical care. Two independent reviewers (R.C.M. and A.M.M.) who were blinded to treatment allocation and placental histology scored the severity and location of brain injury using a validated HIE scoring system22,23, with discrepancies resolved by consensus. A global brain injury score (range 0–138) was assigned by summing the injury severity seen on T1, T2, and diffusion-weighted sequences within 8 brain regions: caudate, putamen/globus pallidus, thalamus, posterior limb of internal capsule, white matter, cortex, brainstem, and cerebellum. Subcortical brain injury was defined as injury to the caudate, putamen, globus pallidus, thalamus, or posterior limb of the internal capsule.
At 12 months of age, motor and cognitive functions were assessed using the Warner Initial Developmental Evaluation of Adaptive and Functional Skills (WIDEA)24 parental questionnaire and the Alberta Infant Motor Scale (AIMS)25 evaluation. We evaluated associations between placental abnormalities, MRI, and other clinical characteristics using χ2, Fisher’s exact, t test, or Wilcoxon rank-sum test as appropriate, with a two-tailed significance level of P < 0.05. We did not perform multivariable analyses due to small sample size. Institutional review board approval was obtained at all hospitals that participated in the NEATO trial.
Results
Forty-seven of the 50 (94%) enrolled infants were born at an outlying hospital prior to being transferred to 1 of the 7 study sites. Thirty-five of the 50 (70%) patients had a placenta that underwent pathologic examination. Patients with no placental examination were more likely to be delivered via spontaneous unassisted vaginal delivery, when compared to patients whose placentas underwent pathologic evaluation (53% vs. 17%, P = 0.01). Severity of encephalopathy, degree of acidosis, and other clinical features were similar between patients who did and did not undergo placental examination (Table 1).
Histologic abnormalities were reported in 19 of the 35 (54%) placental specimens. Chronic abnormalities were the most common finding (N = 13, 37%), including maternal vascular malperfusion (N = 12, 34%), villitis of unknown etiology (N = 4, 11%), and fetal vascular malperfusion (N = 2, 6%). Four (11%) placentas had >1 of these findings, including 1 placenta that exhibited all 3 types of chronic abnormalities. Histologic chorioamnionitis was reported in 9 (26%) placentas. Three (9%) patients exhibited both chronic abnormalities and histologic chorioamnionitis. Among nine mothers with histologic chorioamnionitis, only one received a clinical diagnosis of chorioamnionitis. Conversely, of the three mothers who received a clinical diagnosis of chorioamnionitis, only one had histologic evidence of placental inflammation.
The patients with a placental histologic abnormality of either chronic or acute nature had a lower mean head circumference than those with a normal placental exam (34.0 vs. 35.1 cm, P = 0.03). The presence of a chronic placental abnormality was associated with a smaller head circumference (mean 33.7 vs. 34.9, P = 0.02), lower birth weight (mean 3037 vs. 3458 g, P = 0.02), and a trend toward lower placental weight (mean 445 vs. 521 g, P = 0.07). Pre-eclampsia was also more common in patients with a chronic placental abnormality (23% vs. 0%, P = 0.04). Fetoplacental ratio did not differ between patients with and without a chronic placental abnormality (Table 2).
Neonatal brain MRI was performed in all 35 subjects with available placental pathology at a mean age of 5.1 days (SD 2.4). Global brain injury scores ranged from 0 to 70 (median 4, interquartile range [IQR], 0 to 11) and did not differ between infants with and without placental abnormalities (median 4, IQR 0–12 vs. 6, IQR 2–11, P = 0.51). Subcortical brain injury was less common in those with any placental abnormality than in patients with a normal placenta (26% vs. 69%, P = 0.02, Table 3). Subcortical brain injury was also less common in patients with a chronic placental abnormality (23% vs. 59%, P = 0.08), though numbers were small and did not reach statistical significance. No differences in global or regional brain injury were observed between patients with and without histologic chorioamnionitis.
The 10 patients who were born following a sentinel event had a higher risk of subcortical brain injury than those without this intrapartum complication (80% vs. 32%, P = 0.02). Chronic placental abnormalities were equally common in patients with and without exposure to a sentinel event (50% vs. 56%, P = 0.99). None of the six patients whose placentas showed only acute histologic chorioamnionitis were exposed to a sentinel event. Of the three mothers diagnosed with pre-eclampsia, two suffered a placental abruption sentinel event, and all three were found to have maternal vascular malperfusion on placental histology.
Abnormalities on gross examination included meconium staining (N = 8) and membranous insertion of the umbilical cord (N = 2). Mean placental weight did not differ between patients with and without histologic abnormalities (473 vs. 515 g, P = 0.32) and was not associated with MRI findings. Fetoplacental ratio >90th percentile (12%) and <10th percentile (12%) similarly did not correlate with either histologic or MRI findings. Baseline plasma Epo levels (U/L) did not differ significantly between subjects with and without placental histologic findings (median 27 IQR 0.3–72 vs. 25 IQR 0.3–102, P = 0.81) nor between those with and without chronic placental abnormalities (median 11 IQR 0.3–33 vs. 29 IQR 0.3–113, P = 0.12). At 12 months of age, patients with and without placental histologic abnormalities showed no difference in neurodevelopmental outcomes as measured by the AIMS or WIDEA (Table 3).
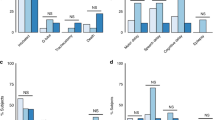
In the parent NEATO trial, we found that patients with HIE who received Epo demonstrated lower global brain injury scores and less subcortical brain injury.5 When stratified by placental histology, we found that Epo treatment was associated with lower global brain injury score (median 2.0 vs. 11.5, P = 0.003) and a lower rate of subcortical brain injury (33% vs. 90%, P = 0.01) only among the 22 patients who had no chronic abnormality on placental examination (Fig. 1). In contrast, among the 13 patients whose placentas harbored a chronic abnormality, Epo imparted no apparent benefit on either global brain injury score (median 4.5 vs. 4.0, P = 0.50) or the frequency of subcortical brain injury (17% vs. 29%, P = 0.99).
We further examined the relationship between chronic placental abnormalities and brain injury within the two treatment groups. In the placebo group, subcortical brain injury was less common in patients with chronic placental abnormalities than those without such findings (29% vs. 90%, P = 0.04). Among patients who received Epo, the rate of subcortical brain injury was similar in those with and without chronic placental findings (17% vs. 33%, P = 0.62).
Discussion
Among patients undergoing hypothermia for moderate-to-severe HIE who were enrolled in a phase II clinical trial, over half exhibited histologic abnormalities on placental examination. The presence of placental abnormalities was associated with a lower rate of subcortical brain injury on neonatal MRI. Furthermore, the response to Epo treatment varied by the presence of placental abnormalities; Epo treatment was associated with less injury on neonatal brain MRI but only in patients whose placentas did not exhibit a chronic histologic change.
Previous studies of newborns with HIE have reported inconsistent relationships between placental histology and brain MRI. Histologic chorioamnionitis has been associated with an increased risk of MRI brain injury in some16 but not all HIE cohorts.20,26 Chronic villitis was not present in any of the 23 infants with HIE14, yet in a larger study of 95 patients, chronic villitis was described in 23% of patients with HIE and was associated with a 12.7-fold increased risk of basal ganglia injury.20
In our HIE cohort, histologic chorioamnionitis and chronic villitis were neither common nor associated with brain injury. Instead, we found a trend towards chronic placental abnormalities being associated with a reduced likelihood of subcortical brain injury. One possible explanation is that placental insufficiency resulting from chronic placental abnormalities leads to the prolonged and partial type of hypoxic insult that is typically associated with a watershed pattern of injury.27 However, the frequency of white matter injury was similar in our patients with and without placental abnormalities. Another hypothesis invokes the concept of ischemic pre-conditioning.28 Animal studies suggest that prior episodes of hypoxia can attenuate injury from subsequent hypoxic–ischemic insults. Thus chronic hypoxia due to placental insufficiency could play a neuroprotective role against hypoxic–ischemic brain injury. Indeed, in some patient reports, low placental weight indicative of potential chronic placental insufficiency has been associated with lower rates of basal ganglia injury after HIE.16,20 However, we were unable to confirm an association between low placental weight and brain injury. Furthermore, Epo levels were not elevated in our patients with chronic placental abnormalities, as might have been expected if ischemic pre-conditioning had resulted from increased fetal hypoxia-inducible factor-1 production.29 Thus the potential role of ischemic pre-conditioning in our patients remains speculative.
Epo is a cytokine that demonstrates potent neuroprotective effects in animal models of HIE, including acute anti-inflammatory and anti-excitotoxic effects.30 In rodent models, Epo increases neurogenesis in injured basal ganglia and cortex31,32, indicating a regenerative effect that occurs days to weeks after hypoxic–ischemic injury. In a rodent model of neonatal ischemia, Epo preserved brain volume and improved sensorimotor function even when the initiation of treatment was delayed until 7 days after injury.33
We found that Epo treatment was associated with less MRI brain injury but not in patients whose placentas harbored a chronic histologic abnormality. There are several potential explanations for this finding. First, it is possible that Epo exerts less neuroprotection in patients who have been exposed to chronic placental disease and an older onset of injury, despite animal data supporting a delayed regenerative effect of this therapy. Alternatively, since presence of a chronic placental abnormality was associated with reduced subcortical brain injury even among patients who received placebo, it is possible that long-standing placental abnormalities afford a baseline degree of neuroprotection against hypoxic–ischemic brain injury, such that patients will suffer minimal injury after receiving hypothermia treatment alone. Finally, our findings may have resulted from confounding and selection bias due to several important study limitations.
First, the small size of this study limits the conclusions that can be drawn. Only 70% of patients enrolled in the NEATO trial had a placental pathology report available, though this percentage is similar to that reported in previous HIE cohorts (56–84%).19,20 Given the small size, we were unable to perform multivariate analyses. We relied exclusively on clinical pathology reports since placental tissue was not available for direct examination, and most placental reports were generated at outlying birth hospitals by pathologists without specific training in placental pathology, using sampling methods and terminology that were not standardized. Of note, our finding that the presence of a chronic placental abnormality was associated with lower birth weight and head circumference suggests that clinical pathology reports can nonetheless produce meaningful findings in research settings.34 The clinical neuroimaging studies were not performed in a standard fashion across sites. Finally, this study pertains only to patients who meet clinical criteria for HIE and not to patients with all causes of NE.35 The clinical criteria for HIE are non-specific, the differential diagnosis of HIE and NE is large, and the multiple antenatal risk factors for both HIE and NE6,36 are beyond the scope of this study.
We enrolled only patients with HIE and no comparison group; thus the underlying etiologies of HIE cannot be evaluated in our study. Recent reports suggest that placental vascular or inflammatory lesions are present in the majority of healthy term deliveries (66–78%).37,38 Three studies19,37,38 that described placental lesions in healthy term infants, including 2 studies19,38 that classified histologic findings using published consensus criteria39, found the following frequencies of histologic findings: villitis of unknown etiology (10–13%), maternal vascular malperfusion (13–19%), fetal vascular malperfusion (10–14%), and acute chorioamnionitis with or without funisitis (10–36%). When early cases of chorioamnionitis, i.e., early acute subchorionitis, were excluded as indicated by the Amsterdam consensus criteria39, the rate of histologic chorioamnionitis with or without funisitis in healthy controls was 10–23%.38,39
Although we are unable to determine causes of HIE in our patients, our findings do suggest that multiple insults occurring at varying times may contribute to the pathogenesis of HIE.6,15 For instance, 3 of the 13 (23%) patients with chronic placental abnormalities also had signs of histologic chorioamnionitis and therefore exhibited both chronic and acute placental lesions. Furthermore, half of our patients exposed to an acute clinical sentinel event also demonstrated underlying placental abnormalities that were chronic in nature. Almost one third of our patients were exposed to an acute sentinel event; this is a similar proportion (36%) to that described in a recent population-based HIE cohort.13 Therefore, subdividing patients into those exposed to an acute sentinel event, acute placental lesion, chronic placental lesion, or a combination of these factors may allow future studies of HIE to better elucidate underlying risk factors and pathogenetic mechanisms.
Despite the limitations of this study, our findings suggest that placental examination can provide important clues to underlying etiology and treatment response. Ideally, when a newborn with HIE is transported to a tertiary care hospital for hypothermia treatment, the placenta should be transported with the patient and be examined by an experienced perinatal pathologist using published consensus criteria.39 However, there are many barriers to obtaining routine placental examinations: placentas are often discarded, there is a paucity of trained placental pathologists, and placental tissue is considered by some insurance companies to belong to the mother, making the placental examination an uncovered medical expense when incurred at a hospital outside of the birth hospital.
In summary, placental data may provide clues to etiology and response to treatment; therefore, placentas should be submitted for examination as standard practice in all HIE trials. In this phase II trial of Epo treatment for HIE, we found that placental abnormalities were associated with a lower risk of subcortical brain injury and that response to Epo treatment was attenuated in patients who harbored a chronic placental abnormality. However, given the small size of this study, these findings require further confirmation. On-going phase III trials40 may provide additional insights into the relationship between placental abnormalities, MRI brain injury, and response to neuroprotective therapies in patients with HIE.
References
Kurinczuk, J. J., White-Koning, M. & Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev. 86, 329–338 (2010).
Graham, E. M., Ruis, K. A., Hartman, A. L., Northington, F. J. & Fox, H. E. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am. J. Obstet. Gynecol. 199, 587–595 (2008).
Black, R. E. et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 375, 1969–1987 (2010).
Shankaran, S. et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N. Engl. J. Med. 366, 2085–2092 (2012).
Wu, Y. W. et al. High-dose erythropoietin and hypothermia for hypoxic-ischemic encephalopathy: a phase II trial. Pediatrics 137, e20160191 (2016).
ACOG (ed) Neonatal Encephalopathy and Neurologic Outcome (AAP, Washington DC, 2014).
Badawi, N. et al. Intrapartum risk factors for newborn encephalopathy: the Western Australian case-control study [see comments]. BMJ 317, 1554–1558 (1998).
Badawi, N. et al. Antepartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ 317, 1549–1553 (1998).
West, C. R. et al. Antenatal antecedents of moderate or severe neonatal encephalopathy in term infants-a regional review. Aust. N. Z. J. Obstet. Gynaecol. 45, 207–210 (2005).
Nelson, K. B. et al. Antecedents of neonatal encephalopathy in the Vermont Oxford Network Encephalopathy Registry. Pediatrics 130, 878–886 (2012).
Hayes, B. C. et al. A case-control study of hypoxic-ischemic encephalopathy in newborn infants at >36 weeks gestation. Am. J. Obstet. Gynecol. 209, 29.e1–29.e19 (2013).
Martinez-Biarge, M., Diez-Sebastian, J., Wusthoff, C. J., Mercuri, E. & Cowan, F. M. Antepartum and intrapartum factors preceding neonatal hypoxic-ischemic encephalopathy. Pediatrics 132, e952–959 (2013).
Parker, S. J., Kuzniewicz, M., Niki, H. & Wu, Y. W. Antenatal and intrapartum risk factors for hypoxic-ischemic encephalopathy in a US birth cohort. J. Pediatr. 203, 163–169 (2018).
Okereafor, A. et al. Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics 121, 906–914 (2008).
Redline, R. W. & O’Riordan, M. A. Placental lesions associated with cerebral palsy and neurologic impairment following term birth. Arch. Pathol. Lab. Med. 124, 1785–1791 (2000).
Wintermark, P., Boyd, T., Gregas, M. C., Labrecque, M. & Hansen, A. Placental pathology in asphyxiated newborns meeting the criteria for therapeutic hypothermia. Am. J. Obstet. Gynecol. 203, 579.e1–579.e9 (2010).
Mir, I. N. et al. Placental pathology is associated with severity of neonatal encephalopathy and adverse developmental outcomes following hypothermia. Am. J. Obstet. Gynecol. 213, e841–847 (2015).
Redline, R. W. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am. J. Obstet. Gynecol. 192, 452–457 (2005).
Vik, T. et al. The placenta in neonatal encephalopathy: a case-control study. J. Pediatr. 202, 77.e3–85.e3 (2018).
Harteman, J. C. et al. Placental pathology in full-term infants with hypoxic-ischemic neonatal encephalopathy and association with magnetic resonance imaging pattern of brain injury. J. Pediatr. 163, 968.e2–995.e2 (2013).
Redline, R. W., Boyd, P. A. & Roberts, D. J. Placental and Gestational Pathology (Cambridge University Press, Cambridge, UK, 2018).
Bednarek, N. et al. Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology 78, 1420–1427 (2012).
Trivedi, S. B. et al. A validated clinical MRI injury scoring system in neonatal hypoxic-ischemic encephalopathy. Pediatr. Radiol. 47, 1491–1499 (2017).
Msall, M. E. Measuring functional skills in preschool children at risk for neurodevelopmental disabilities. Ment. Retard. Dev. Disabil. Res. Rev. 11, 263–273 (2005).
Piper, M. C., Pinnell, L. E., Darrah, J., Maguire, T. & Byrne, P. J. Construction and validation of the Alberta Infant Motor Scale (AIMS). Can. J. Public Health 83(Suppl 2), S46–50 (1992).
Frank, C. M. et al. Placental pathology and outcome after perinatal asphyxia and therapeutic hypothermia. J. Perinatol. 36, 977–984 (2016).
Martinez-Biarge, M. et al. White matter and cortical injury in hypoxic-ischemic encephalopathy: antecedent factors and 2-year outcome. J. Pediatr. 161, 799–807 (2012).
Gustavsson, M., Anderson, M. F., Mallard, C. & Hagberg, H. Hypoxic preconditioning confers long-term reduction of brain injury and improvement of neurological ability in immature rats. Pediatr. Res. 57, 305–309 (2005).
Sheldon, R. A., Lee, C. L., Jiang, X., Knox, R. N. & Ferriero, D. M. Hypoxic preconditioning protection is eliminated in HIF-1alpha knockout mice subjected to neonatal hypoxia-ischemia. Pediatr. Res. 76, 46–53 (2014).
Wu, Y. W. & Gonzalez, F. F. Erythropoietin: a novel therapy for hypoxic-ischaemic encephalopathy? Dev. Med. Child Neurol. 57(Suppl 3), 34–39 (2015).
Gonzalez, F. F. et al. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Dev. Neurosci. 29, 321–330 (2007).
Gonzalez, F. F. et al. Erythropoietin increases neurogenesis and oligodendrogliosis of subventricular zone precursor cells after neonatal stroke. Stroke 44, 753–758 (2013).
Larpthaveesarp, A., Georgevits, M., Ferriero, D. M. & Gonzalez, F. F. Delayed erythropoietin therapy improves histological and behavioral outcomes after transient neonatal stroke. Neurobiol. Dis. 93, 57–63 (2016).
Grether, J. K. et al. Reliability of placental histology using archived specimens. Paediatr. Perinat. Epidemiol. 13, 489–495 (1999).
McIntyre, S., Badawi, N., Blair, E. & Nelson, K. B. Does aetiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy influence the outcome of treatment? Dev. Med. Child Neurol. 57(Suppl 3), 2–7 (2015).
Aslam, S., Strickland, T. & Molloy, E. J. Neonatal encephalopathy: need for recognition of multiple etiologies for optimal management. Front. Pediatr. 7, 142 (2019).
Romero, R. et al. The frequency and type of placental histologic lesions in term pregnancies with normal outcome. J. Perinat. Med. 46, 613–630 (2018).
Zhou, Y. Y., Ravishankar, S., Luo, G. & Redline, R. W. Diagnostic Yield by Indication for Submission of Singleton Placentas from Term Births. (Society for Pediatric Pathology Interim Meeting, East Lansing, MI, 2018).
Khong, T. Y. et al. Sampling and definitions of placental lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 140, 698–713 (2016).
Juul, S. E. et al. High-dose erythropoietin for asphyxia and encephalopathy (heal): a randomized controlled trial - background, aims, and study protocol. Neonatology 113, 331–338 (2018).
Acknowledgements
The study data were collected and managed by using REDCap electronic data capture tools hosted at University of California, San Francisco. We thank all the patients, families, and bedside nurses who participated in this study. This study was funded by the Thrasher Research Fund.
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation: Y.W.W., A.M.G., T.C., S.B.M., F.F.G., D.E.M., S.E.J., A.M.M., K.V.M., R.C.M., R.W.R. Drafting the article or revising it critically for intellectual content: Y.W.W., A.M.G., R.W.R., D.M., R.M., S.M., T.C., S.J., K.V.M. Final approval of the version submitted: Y.W.W., A.M.G., T.C., S.B.M., F.F.G., D.E.M., S.E.J., A.M.M., K.V.M., R.C.M., R.W.R.
Corresponding author
Ethics declarations
Competing interests
Y.W.W. and R.W.R. have provided expert consultation on cases related to cerebral palsy and HIE. The other authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, Y.W., Goodman, A.M., Chang, T. et al. Placental pathology and neonatal brain MRI in a randomized trial of erythropoietin for hypoxic–ischemic encephalopathy. Pediatr Res 87, 879–884 (2020). https://doi.org/10.1038/s41390-019-0493-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0493-6