Abstract
Mutations in each of the five eucaryotic initiation factor 2B (eIF2B) subunits have been found in leukodystrophies of various severity: Cree leukoencephalopathy, childhood ataxia with central hypomyelination/leukodystrophy with vanishing white matter and ovarioleukodystrophy. A continuum was observed from fatal infantile forms to adult forms without neurological deterioration. Disease severity was found to correlate with the age at disease onset and the specific amino-acid substitution. In order to analyze the functional consequences of eIF2B mutations, we measured the guanine nucleotide exchange factor (GEF) activity of eIF2B in transformed lymphocytes from 30 affected patients carrying mutations in eIF2B compared to 10 unaffected heterozygotes and 22 controls without eIF2B mutations. A significant decrease of 20–70% in GEF activity was observed in all mutated cells. The severity of this decrement of GEF activity correlated with age at onset of the disease. These results suggest that a deficiency in GEF activity underlies the encephalopathy associated eIF2B-related disease. Our study demonstrates that the evaluation of the GEF activity in transformed lymphocytes represents an interesting alternative test to the systematic screening of the five EIF2B genes. This relevant cellular model may also be used to test the functional impact of different molecules on the GEF activity for future therapeutic strategies.
Similar content being viewed by others
Introduction
Mutations in the eucaryotic initiation factor 2B (eIF2B) have been reported in patients with leukodystrophies of various severity. Mutations in each of the five subunits eIF2Bα, β, γ, δ and ɛ have been initially found (genes EIF2B1–EIF2B5 [MIM 606686], [MIM 606454]), [MIM 606272], [MIM 606687], and [MIM 603945]) in patients with leukoencephalopathy with vanishing white matter (VWM),1, 2 also described as childhood ataxia with central hypomyelination (CACH) syndrome (CACH/WVM [MIM 603896]).3 This syndrome, with autosomal recessive inheritance, has an onset most often between 2 and 5 years of age. Affected individuals experience progressive neurological deterioration including ataxia, spasticity, exacerbated by episodes of fever or trauma of the head. Magnetic resonance imaging (MRI) shows a diffuse involvement of the cerebral hemispheric white matter, with CSF-like signal intensity related to rarefaction and cystic degeneration of the white matter.3, 4, 5, 6 Subsequently, eIF2Bɛ mutations were found in fatal infantile forms described as Cree leukoencephalopathy (CLE),7, 8 a severe variant of CACH/VWM9, 10 and more recently in congenital forms.11 At the opposite end of the clinical spectrum, eIF2B mutations were also found in adult-onset forms associated with ovarian failure (ovarioleukodystrophy), and a slow or absent neurological deterioration12 leading to the concept of eIF2B-related disorders with a wide continuum of clinical severity. In a large cohort of 85 eIF2B-mutated patients, we demonstrated that disease severity is correlated to age at disease onset and to the specific amino-acid substitution.13
eIF2B converts the protein synthesis initiation factor 2 (eIF2) from an inactive GDP-bound form to an active eIF2-GTP complex owing to its guanine nucleotide exchange factor (GEF) activity. This active complex plays an important role in the initiation of messenger RNA translation, allowing the formation of the 43S preinitiation complex, a precursor of the active 80S ribosome–mRNA complex. Regeneration of active eIF2 is essential for continued protein synthesis, and eIF2B plays a key role in its regulation by promoting the release of GDP from eIF2.14
In order to analyze the functional consequences of eIF2B mutations, we measured for the first time the GEF activity of eIF2B in transformed lymphocytes from 30 affected patients carrying mutations in eIF2B, and exhibiting various types of disease severity, compared to 10 unaffected heterozygotes and 22 controls without eIF2B mutations. We hypothesized that although disruption of the ubiquitously expressed eIF2B causes mainly a brain disease, its dysfunction can be demonstrated in non-neural cells.
Materials and methods
Selection of patients
The 30 affected patients carrying mutations in eIF2B and representative of the clinical spectrum severity (Table 1) were selected from our series of 85 patients previously reported.13 The 10 healthy heterozygote patients were relatives (parents or siblings) of affected patients (Table 1).
The 22 controls were healthy, age- and sex-matched individuals. No mutations were found in the five patients completely screened for mutations by direct sequencing of the five eIF2B genes. In the other patients, sequences of all exons containing reported mutations2, 13 were normal.
Establishment of transformed lymphocytes
In order to have large quantity of lymphocytes, lymphoblastoid cell lines were obtained by immortalizing patients'peripheral blood lymphocytes with Epstein–Barr virus according to classical procedures.15
Measurement of eIF2B GEF activity
To determine the GEF activity of eIF2B, we adapted the GDP dissociation assay described by Kimball et al.16 This assay is a direct measurement of eIF2B GEF activity since, in the presence of Mg2+, the rate-limiting step of the nucleotide exchange reaction is GDP dissociation from eIF2. One million cells from patient transformed lymphocytes were lysed in 100 μl of ice-cold extraction buffer (45 mM HEPES, pH 7.4, 0.375 mM magnesium acetate, 0.075 mM EDTA, 95 mM potassium acetate, 2.5 mg/ml digitonin, microcystine and 10% (v/v) glycerol). The lysates were centrifuged at 10 000 g for 10 min at 4°C and the resulting supernatants were assayed immediately for the guanine nucleotide exchange activity of eIF2B. Volumes of 60 μl of the lysate supernatants were added with 2 mM Mg2+ to 100 μl assay buffer (62.5 mM MOPS, pH 7.4, 125 mM KCl, 1.25 mM dithiothreitol, and 0.2 mg/ml bovine serum albumin) containing a 100-fold excess of GDP, subsequently followed by 1–2 pmol of radiolabeled binary eIF2-[3H]GDP complex (eIF2 purified from rat liver: 1.38 μg/assay). The mixture was then incubated at 30°C for 0, 2, 4 or 6 min. The exchange reaction was measured as a linear decrease in the eIF2-mediated binding of [3H]GDP to nitrocellulose filters with time. The slope of each reaction was used to represent GEF activity. Each assay was carried out in triplicate.
Measurement of eIF2alpha phosphorylation
Proteins (60 μg) extracted from transformed lymphocytes cells with RIPA buffer (50 mM Tris HCl pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 50 mM NaF, 2 mM NaVO4, 0.1 μ M okadaic acid, 25 mM beta-glycerophosphate, 1 mM PMSF, protease inhibitors (Sigma-Aldrich, Protease inhibitor cocktail for general use)) were loaded per well. The relative amount of eIF2α in the phosphorylated form was quantitated by protein immunoblot analysis using an affinity-purified antibody that specifically recognizes eiF2 phosphorylated at Serine 51 (Phospho-eIF2alpha (Ser51), Cell Signaling Technology). The total amount of eIFalpha in the samples was determined by reprobing the blot with a monoclonal that recognizes equally the phosphorylated and unphosphorylated forms of eIF2alpha (adapted from Kimball et al.17).
Statistics
Data are expressed as mean±SEM. Analysis of variances in GEF activities were performed by one-way ANOVA. Correlation tests and regression were evaluated using the statistical software STATA® (StataCorp LP, Version 6.0).
Results
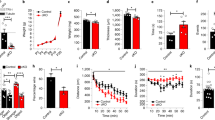
The eIF2B GEF activity was measured in transformed lymphocytes from 30 affected patients exhibiting various types of eIF2B mutations and different ages at disease onset (mean age=5.39 39±4.29 years, range 0.8–17). These activities were compared to those of 22 age-matched wild-type controls (mean age=8.6±6.5 years, range 0.5–25), and to 10 age-matched unaffected heterozygotes (Table 1). GEF activities measured in transformed lymphocytes from the 22 nonmutated controls were not statistically different (99.6±3.5%). A significant decrease in the GEF activity (P<0.01) was observed in cells from affected mutated patients compared to nonmutated cells (mean decrements=41%, range 20–70% of the control value) (Figure 1). No statistical significant difference was observed between control and heterozygote cells (mean 96.7±3%). In addition, the GEF activity measured on transformed lymphocytes from affected patients correlated with the age at disease onset (P<0.01, correlation coefficient r=0.51) (Figure 2). This correlation remained highly significant when the 18 patients with a disease onset before 5 years of age were considered (P<0.01, r=0.59), but correlation was not significant for the 12 patients with disease onset after 5 years of age (r=0.37). To rule out the role of phosphorylated eIF2α on GEF eIF2B activity decrease, we determined the amount of phosphorylated and unphosphorylated forms of eIF2α in eight representative cells from patients with eIF2B mutations (patients 1036, 76-1, 370-1, 435, 823, 571-1, 291, 359) in comparison to controls (Figure 1c comparing three controls with patients 1036, 291 and 359). We found no significant difference in the amount of total eIF2α and eIF2α phosphorylated forms between controls and patients with eIF2B mutations.
Decrement of GEF activity in mutated cells, independent of eIF2α phosphorylation. (a) Significant decrement of GEF activity in cells from affected patients compared to nonmutated or heterozygote ones. eIF2B GEF activity in extracts of transformed lymphocytes from 30 eIF2B-mutated and affected patients (Mut), 10 heterozygote nonaffected patients (Het) and 22 nonmutated patients (non-mut). The guanine nucleotide exchange activity was measured as the exchange of [3H]GDP bound to eIF2 for nonradiolabeled GDP over time. The slope corresponding to the linear decrease in [3H]GDP bound to eIF2 over time was used for comparisons. The results for mutant cells are expressed as a percentage of GEF activity of control cells and represent three independent experiments. *Significantly different (P<0.001) from the heterozygous and nonmutated response. (b) Examples of nucleotide exchange activity shown as picomoles of GDP exchanged. GEF activity was measured in cells from three representative patients with eIF2B mutations: patients 736 (squares), 928 (triangles), 1036 (circles) and compared to control cells (rhombuses). (c) Amounts of total eIF2α and phosphorylated eIF2α in cells from patients with mutations in eIF2B in comparison to age-matched controls are identical.
Correlation between GEF activity and age at disease onset. eIF2B GEF activity, in % control value, of the 30 eIF2B-mutated patients, correlates with age at disease onset (r (correlation coefficient)=0.516, P<0.01). This correlation could be modeled with a logarithmic curve whose equation is: y=9.6 Ln(x)+45.9.
Discussion
We demonstrated for the first time that measurement of the GEF activity of eIF2B is possible on human transformed lymphocytes, and that this activity is decreased in cells from patients homozygous or compound heterozygotes for eIF2B mutations. No abnormalities were found in heterozygotes, in agreement with their normal clinical and MRI presentations. These immortalized mutated lymphocytes are stable, easy to amplify and to preserve, even after patients' death, and offer a good model to study functional consequences of eIF2B mutations. The amount of reduced GEF activity correlated with the age at disease onset, particularly in patients with disease onset before 5 years of age and GEF activity <50%. Absence of correlation for patients with later disease onset and higher GEF activity, suggests that other factors can modulate the effect of mutations on the phenotype.13
The catalytic subunit of eIF2B is the epsilon subunit where the majority of the mutations were found.18, 19 A decrease in GEF activity according to the type of eIFBɛ mutations has been also reported in yeast.14 In this report, mutations in the other subunits were found to reduce eIF2B activity as well. Mutations in one of the five eIF2B subunits would decrease the rate of GTP/GDP exchange due to conformational changes or by preventing a particular subunit from binding to the others to form the holoenzyme. Although the epsilon subunit of eIF2B is the only one that exhibits catalytic activity, alone it only has approximately 5% as much activity as the holocomplex. In particular, the beta, gamma, and delta subunits are required for maximal activity in mammals.14 Thus, mutations in any of the subunits except the alpha could have dramatic effects on eIF2B activity if it affects the interaction of the epsilon subunit with any of the three regulatory subunits.
The GEF activity of eIF2B is a key control point for eukaryotic protein synthesis particularly in response to cellular stresses.18 In response to stress, the phosphorylated form of eIF2, which has a much higher affinity for eIF2B than the unphosphorylated form, acts as a competitive inhibitor of eIF2B activity and results in a rapid reduction in the rate of protein synthesis, which avoids large intracellular accumulation of denatured proteins.19, 14, 20 In patients with eIF2B mutations, a high susceptibility to cellular stress is suggested by the acute neurological deterioration observed after minor head trauma or banal viral infections. However, we found no difference in eIF2B activity nor in the amount of eIF2α phosphorylation in mutated heat-shock stressed cells (1 h at 42°C) versus normal ones (personal communication). Further analyses are needed to study the effect of this decrement in eIF2B activity in response to different stress conditions.
The predominant susceptibility of the cerebral white matter to eIF2B dysfunction remains to be elucidated. eIF2B dysfunction in humans may cause abnormal glial cells development, as suggested by the recent description of congenital forms of eIF2B-related disorders,11 and the similar findings in a mouse knockout model for a transcriptional regulator of heat-shock gene expression (Hsf2).21 This abnormal development of the white matter would increase susceptibility of cells containing mutated eIF2B to cellular stress with subsequent progressive neurological deterioration and cavitation. For patients with a severe decrease in eIF2B activity (age at disease onset <5 years), eIF2B mutations may supersede environmental or other genetic factors to precipitate white-matter consequences of cellular stress leading to a rather homogenous clinical presentation. On the other hand, in milder forms with a disease onset after 5 years, environmental or other genetic factors would play a role that could explain the variability observed in the onset of the neurological deterioration even within individuals of the same family. For example, the two siblings (patients 76-1 and 76-2) who had similar decrement in GEF activity, had a disease onset at, 17 and 7 years of age respectively (Table 1).
In conclusion, all eIF2B-mutated patients exhibited a decreased GEF activity of eIF2B. Accordingly, this assay could be developed as a biochemical screening test to select patients eligible for sequencing of the five EIF2B genes. This eIF2B assay may also be used to test the functional impact of different molecules on the GEF activity for future therapeutic strategies.
References
Leegwater PA, Vermeulen G, Konst AA et al: Subunits of the translation initiation factor eIF2B are mutant in leukoencephalopathy with vanishing white matter. Nat Genet 2001; 29: 383–388.
van der Knaap MS, Leegwater PA, Konst AA et al: Mutations in each of the five subunits of translation initiation factor eIF2B can cause leukoencephalopathy with vanishing white matter. Ann Neurol 2002; 51: 264–270.
Schiffmann R, Moller JR, Trapp BD et al: Childhood ataxia with diffuse central nervous system hypomyelination. Ann Neurol 1994; 35: 331–340.
van der Knaap MS, Barth PG, Gabreels FJ et al: A new leukoencephalopathy with vanishing white matter. Neurology 1997; 48: 845–855.
Rodriguez D, Gelot A, della Gaspera B et al: Increased density of oligodendrocytes in childhood ataxia with diffuse central hypomyelination (CACH) syndrome: neuropathological and biochemical study of two cases. Acta Neuropathol 1999; 97: 469–480.
Wong K, Armstrong RC, Gyure KA et al: Foamy cells with oligodendroglial phenotype in childhood ataxia with diffuse central nervous system hypomyelination syndrome. Acta Neuropathol 2000; 100: 635–646.
Black DN, Booth F, Watters GV et al: Leukoencephalopathy among native Indian infants in northern Quebec and Manitoba. Ann Neurol 1988; 24: 490–496.
Fogli A, Dionisi-Vici C, Deodato F, Bartuli A, Boespflug-Tanguy O, Bertini E : A severe variant of childhood ataxia with central hypomyelination/vanishing white matter leukoencephalopathy related to EIF2B5 mutation. Neurology 2002; 12: 1966–1999.
Francalanci P, Eymard-Pierre E, Dionisi-Vici C et al: Fatal infantile leukodystrophy, a severe variant of CACH/VWM syndrome, allelic to chromosome 3q27. Neurology 2001; 57: 265–270.
Fogli A, Wong K, Eymard-Pierre E et al: Cree leukoencephalopathy and CACH/VWM disease are allelic at the EIF2B5 locus. Ann Neurol 2002; 52: 506–510.
van der Knaap MS, van Berkel CG, Herms J et al: eIF2B-related disorders: antenatal onset and involvement of multiple organs. Am J Hum Genet 2003; 73: 1199–1207.
Fogli A, Rodriguez D, Eymard-Pierre E et al: Ovarian failure related to eukaryotic initiation factor 2B mutations. Am J Hum Genet 2003; 72: 1544–1550.
Fogli A, Schiffmann R, Bertini E et al: The effect of genotype on the natural history of eIF2B-related leukodystrophies. Neurology 2004, in press.
Gomez E, Pavitt GD : Identification of domains and residues within the epsilon subunit of eukaryotic translation initiation factor 2B (eIF2Bepsilon) required for guanine nucleotide exchange reveals a novel activation function promoted by eIF2B complex formation. Mol Cell Biol 2000; 20: 3965–3976.
Neitzel H : A routine method for the establishment of permanent growing lymphoblastoid cell lines. Hum Genet 1986; 73: 320–336.
Kimball SR, Everson WV, Flaim KE, Jefferson LS : Initiation of protein synthesis in a cell-free system prepared from rat hepatocytes. Am J Physiol 1989; 256: C28–C34.
Kimball SR, Horetsky RL, Ron D, Jefferson LS, Harding HP : Mammalian stress granules represent sites of accumulation of stalled translation initiation complexes. Am J Physiol Cell Physiol 2003; 284: C273–C284.
Webb BL, Proud CG : Eukaryotic initiation factor 2B (eIF2B). Int J Biochem Cell Biol 1997; 29: 1127–1131.
Kimball SR, Mellor H, Flowers KM, Jefferson LS : Role of translation initiation factor eIF-2B in the regulation of protein synthesis in mammalian cells. Prog Nucleic Acid Res Mol Biol 1996; 54: 165–196.
Clemens MJ : Initiation factor eIF2 alpha phosphorylation in stress responses and apoptosis. Prog Mol Subcell Biol 2001; 27: 57–89.
Kallio M, Chang Y, Manuel M et al: Brain abnormalities defective meiotic chromosome synapsis and female subfertility in HSF2 null mice. EMBO J 2002; 21: 2591–2601.
Acknowledgements
We gratefully acknowledge the participation of the patients' families. We thank Sylvie Mordier (UNMP, INRA Theix, France) for her help in Western blotting. We thank M Pineda, M Troncoso, G Uziel, R Surtees, D Pugin and MP Chaunu for patient referral. We thank E Eymard-Pierre and CR Kaneski for technical help in processing blood samples. This work was supported by grant from the European Leukodystrophy Association (ELA), the Fondation pour la Recherche Médicale (FRM, ARS 2000), the National Institutes of Health (SRK, DK13499), and the Jean Pierre and Nancy Boespflug myopathic research foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fogli, A., Schiffmann, R., Hugendubler, L. et al. Decreased guanine nucleotide exchange factor activity in eIF2B-mutated patients. Eur J Hum Genet 12, 561–566 (2004). https://doi.org/10.1038/sj.ejhg.5201189
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejhg.5201189
Keywords
This article is cited by
-
Mammalian integrated stress responses in stressed organelles and their functions
Acta Pharmacologica Sinica (2024)
-
Adult-onset leukodystrophy with vanishing white matter: a case series of 19 patients
Journal of Neurology (2023)
-
Genotypic and phenotypic characteristics of juvenile/adult onset vanishing white matter: a series of 14 Chinese patients
Neurological Sciences (2022)
-
Foetal onset of EIF2B related disorder in two siblings: cerebellar hypoplasia with absent Bergmann glia and severe hypomyelination
Acta Neuropathologica Communications (2020)
-
Mitochondrial aminoacyl-tRNA synthetase disorders: an emerging group of developmental disorders of myelination
Journal of Neurodevelopmental Disorders (2019)