-
PDF
- Split View
-
Views
-
Cite
Cite
Henriette J. Tschampa, Kai Kallenberg, Horst Urbach, Bettina Meissner, Claudia Nicolay, Hans A. Kretzschmar, Michael Knauth, Inga Zerr, MRI in the diagnosis of sporadic Creutzfeldt–Jakob disease: a study on inter-observer agreement, Brain, Volume 128, Issue 9, September 2005, Pages 2026–2033, https://doi.org/10.1093/brain/awh575
Close - Share Icon Share
Abstract
According to the current WHO criteria, technical investigations included in the clinical diagnosis of sporadic Creutzfeldt–Jakob disease (sCJD) are electroencephalogram (EEG) and CSF-analysis for 14-3-3 proteins. MRI is not a criterion for the diagnosis of sCJD, although typical changes have been described. We investigated the reliability of MRI in the sCJD diagnosis, evaluated MRI sequences and compared MRI with EEG and 14-3-3. This study includes 193 consecutive suspected sCJD patients who had been referred to the German CJD Surveillance Unit from 2001 to 2003. Three observers independently analysed MRI scans, blinded to clinical data. MRI was rated as ‘typical for sCJD’ if increased signal intensity was detected in the caudate nucleus and putamen. We analysed 442 MRI scans [184 T2-weighted sequences, 132 fluid attenuated inversion recovery (FLAIR) sequences, 75 diffusion-weighted sequences and 51 proton-density weighted sequences]. Inter-observer agreement was 123 of 193 patients or 63.7% (overall κ = 0.53). Sensitivity of MRI in clinically probable or autopsy-proven sCJD was 59.7% for Observer 1, 58.3% for Observer 2 and 70.8% for Observer 3; specificity was high (84.2, 89.5 and 81.6%, respectively). Diffusion-weighted sequences best showed the pathologic changes, followed by FLAIR. Periodic sharp and slow wave complexes were detected in the EEG in 32% (sensitivity), the 14-3-3 proteins in CSF were elevated in 91%. We conclude that the detection of hyperintense basal ganglia in MRI helps to improve the clinical diagnosis, and therefore, we propose to incorporate MRI in the diagnostic criteria for sCJD.
Introduction
Creutzfeldt–Jakob disease (CJD) is a uniformly fatal neurodegenerative disorder caused by the accumulation of an abnormal form of the human prion protein PrPSc in the brain (Masters et al., 1979; Kretzschmar et al., 1996). For a definitive diagnosis, brain biopsy or autopsy is required (definite CJD) (Kretzschmar et al., 1996). Clinical diagnosis is based on the combination of rapidly progressive dementia, myoclonus and multifocal neurological dysfunction associated with an electroencephalogram (EEG) showing generalized periodic sharp wave complexes (PSWCs) (Steinhoff et al., 1996) and/or a positive 14-3-3 protein test in CSF (Zerr et al., 1998) [probable sporadic CJD (sCJD)] (Masters et al., 1979; World Health Organization, 1998; Zerr et al., 2000a). Patients with the clinical signs of sCJD but without the EEG and CSF abnormalities are clinically classified as possible sCJD (World Health Organization, 1998). The PSWCs in EEG are present in about two-thirds of sCJD patients (sensitivity 66%) and have a reported specificity of 74% (Steinhoff et al., 1996; Zerr et al., 2000a). Sensitivity of 14-3-3 for the diagnosis of sCJD is higher at 94% and specificity is 84% (Zerr et al., 1998; Zerr et al., 2000a). CJD can be diagnosed only after the exclusion of other illnesses or conditions that would explain the clinical signs and symptoms, changes in the EEG or detection of 14-3-3 protein in CSF. Most patients with suspected CJD are studied with MRI during the course of the disease to exclude other possibly treatable diseases or to support the diagnosis of CJD. Although it is already known that MRI shows characteristic findings in CJD (Finkenstaedt et al., 1996; Urbach et al., 1998; Schroter et al., 2000; Collie et al., 2001; Urbach et al., 2001; Shiga et al., 2004), its potential for clinical diagnosis has not been clarified.
Initially reported on T2-weighted images and proton-density (PD) weighted images, MRI of sCJD patients often shows high signal in the head of the caudate nucleus and in the putamen as compared with the thalamus and the cerebral cortex (Schroter et al., 2000). These changes have a reported sensitivity of 67% and a specificity of 93% for the diagnosis (Schroter et al., 2000). Hyperintense signal changes can also occur in the thalamus, but in sCJD they are usually less prominent than those in the putamen or the caudate nucleus. In addition, there is high signal in the cerebral cortex in some cases. With the introduction of the new sequences fluid attenuated inversion recovery (FLAIR) and diffusion-weighted (DW) imaging, these signal changes are more easily identified (Bahn et al., 1997; Kropp et al., 2000; Sellars et al., 2002; Shiga et al., 2004). DW imaging shows decreased apparent diffusion coefficient values in the affected areas, most probably because of the characteristic neuropathological spongiform neuropil changes (Bahn and Parchi, 1999; Tschampa et al., 2003).
Patients with the new variant of CJD (vCJD) show bilateral increased signal on MRI in the pulvinar thalami (relative to the grey matter of other basal ganglia and the cerebral cortex) with a reported sensitivity of 78% and a specificity of 100% (Zeidler et al., 2000; Collie et al., 2003). This MRI finding was incorporated in the current clinical diagnostic criteria for probable vCJD (Will et al., 2000).
The aims of our study were (i) to investigate the sensitivity, specificity and inter-observer agreement of the typical MRI findings in an unselected group of sCJD patients; (ii) to define the diagnostic value of currently used MRI techniques and (iii) to compare the MRI diagnoses with the established technical investigations, i.e. EEG (Steinhoff et al., 1996) and 14-3-3 protein analysis (Zerr et al., 1998).
Methods
Patients
We studied 193 consecutive suspected sCJD patients who had been notified to the German CJD Surveillance study from June 2001 to July 2003. The hard copies of the MRI exams had been collected from institutions all over Germany.
On the basis of the clinical information and on-site exam by a neurologist of the German CJD Surveillance Unit (University of Göttingen), the patients were classified according to the diagnostic criteria (World Health Organization, 1998; Zerr et al., 1998) as clinically probable, possible or ‘not’ CJDs. Autopsy was performed, if available, in the German Reference Center for spongiform encephalopathies (University of München) (Kretzschmar et al., 1996). After autopsy, patients were reclassified in definite sCJD or ‘not CJD’.
Definite sCJD was proven by autopsy in 60 cases. Clinically probable sCJD was the diagnosis in 84 patients and clinically possible sCJD in 11 patients; ‘not sCJD’ was the clinical or pathological diagnosis in 38 cases.
MRI analysis
A total of 442 MRI scans of 193 patients were analysed (184 T2-weighted, 132 FLAIR, 75 DW and 51 PD weighted studies). Field strengths of the MR scanners were 1.5 T in 89, 1.0 T in 75, 0.5 T in 13 cases. In 16 cases the MR scanner was not identifiable on the hard copies. All hard copies that had been provided by the referring hospitals, e.g. T1-weighted and contrast enhanced scans, were additionally looked at to rule out different diagnoses.
Three observers (H.T., K.K., H.U.) independently analysed the MRI scans. They only knew that CJD was suspected, otherwise they were blinded to all clinical data. Considering all available sequences, the observers judged all the MRI scans taken together as a whole for each patient either as ‘typical for sCJD’, as ‘not typical for sCJD’ or as ‘non-diagnostic’. The exam was considered as ‘typical for sCJD’ if increased signal was detected in the caudate nucleus and putamen in one or more of the evaluated sequences and if MRI did not suggest another diagnosis (such as stroke, lymphoma or encephalitis). Increased signal in the caudate nucleus and putamen was defined as hyperintense signal intensity relative to the normal appearing thalamus and cerebral cortex. Unilateral changes were accepted if there was no evidence of another underlying disease (e.g. infarction). If the hyperintense signal changes were not present, the MRI was rated as ‘not typical for sCJD’. Exams, which were of insufficient image quality owing to motion artefacts or low quality hard copies, were rated as ‘non-diagnostic’. In patients examined more than once (n = 15 cases) the MRI at the time of clinical diagnosis was used for classification purposes.
Inter-observer agreement in MRI analysis
To determine the degree of agreement between the observers, the kappa coefficient (κ) was calculated for each pair of observers as well as the overall κ (all observers combined) (Fleiss, 1971).
Sensitivity, specificity and positive predictive value of MRI
The gold standard for the calculation of sensitivity and specificity for the MR diagnosis were the autopsy results (German Reference Center for spongiform encephalopathies, München) (Kretzschmar et al., 1996) or the clinical classification according to the criteria (World Health Organization, 1998; Zerr et al., 1998). As in earlier studies, clinically probable and autopsy-proven ‘definite’ cases were considered together as ‘sCJD’. ‘Possible’ cases (n = 11) were not included in the calculation of sensitivity or specificity because of diagnostic uncertainty. Sensitivity, specificity and positive predictive values (with 95% confidence interval) (Newcombe and Altman, 2000) were calculated individually for each observer.
Comparison of MR sequences
If the MRIs of a patient were considered as ‘typical’ for sCJD and the clinical or pathological classification was probable or definite sCJD, the available T2-weighted, FLAIR, PD and DW images were graded according to how clearly they showed the pathological changes (1 = best, 2 = second best, 3 = third best and 4 = worst). The grading was performed individually for each patient and independently by each observer. The number of sequences per patient differed. Only a minority of patients had all four sequences. This depended on the images provided by the referring hospitals. All scans were analysed at the same time so that signal abnormalities seen in one sequence were then searched for in the other sequences also.
EEG and 14-3-3 protein
Analysis of EEG (Steinhoff et al., 1996; Zerr et al., 2000a) and 14-3-3 proteins (Zerr et al., 1998; Schroter et al., 2000; Zerr et al., 2000a, b) were carried out in the German CJD Surveillance Unit, Göttingen, as previously described.
Results
MRI analysis
In 60 patients with neuropathologically proven sCJD, 34, 38 and 39 MRI scans were rated as ‘typical’ for CJD (hyperintense basal ganglia) in our study. The rate of ‘typical MRI’ was similar in ‘probable CJD’. However, out of 38 ‘not CJD’ patients, 31, 32 and 34 were rated as ‘not typical’ (Tables 1 and 3). Written reports on the MRI interpretation in the centre of first attendance were available in 106 out of 144 patients with definite or probable CJD. Of these, high signal was described in the basal ganglia only in 30 patients (28%).
MRI analysis
| . | Observer 1 . | . | . | Observer 2 . | . | . | Observer 3 . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Typical . | Not typical . | Non-diagnostic . | Typical . | Not typical . | Non-diagnostic . | Typical . | Not typical . | Non-diagnostic . | ||||||
| Definite (n = 60) | 39 | 15 | 6 | 34 | 21 | 5 | 38 | 21 | 1 | ||||||
| Probable (n = 84) | 47 | 33 | 4 | 50 | 28 | 6 | 64 | 17 | 3 | ||||||
| Possible (n = 11) | 4 | 7 | – | 5 | 5 | 1 | 5 | 5 | 1 | ||||||
| ‘Not CJD’ (n = 38) | 4 | 32 | 2 | 4 | 34 | – | 7 | 31 | – | ||||||
| Total (n = 193) | 94 | 87 | 12 | 93 | 88 | 12 | 114 | 74 | 5 | ||||||
| . | Observer 1 . | . | . | Observer 2 . | . | . | Observer 3 . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Typical . | Not typical . | Non-diagnostic . | Typical . | Not typical . | Non-diagnostic . | Typical . | Not typical . | Non-diagnostic . | ||||||
| Definite (n = 60) | 39 | 15 | 6 | 34 | 21 | 5 | 38 | 21 | 1 | ||||||
| Probable (n = 84) | 47 | 33 | 4 | 50 | 28 | 6 | 64 | 17 | 3 | ||||||
| Possible (n = 11) | 4 | 7 | – | 5 | 5 | 1 | 5 | 5 | 1 | ||||||
| ‘Not CJD’ (n = 38) | 4 | 32 | 2 | 4 | 34 | – | 7 | 31 | – | ||||||
| Total (n = 193) | 94 | 87 | 12 | 93 | 88 | 12 | 114 | 74 | 5 | ||||||
MRI analysis
| . | Observer 1 . | . | . | Observer 2 . | . | . | Observer 3 . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Typical . | Not typical . | Non-diagnostic . | Typical . | Not typical . | Non-diagnostic . | Typical . | Not typical . | Non-diagnostic . | ||||||
| Definite (n = 60) | 39 | 15 | 6 | 34 | 21 | 5 | 38 | 21 | 1 | ||||||
| Probable (n = 84) | 47 | 33 | 4 | 50 | 28 | 6 | 64 | 17 | 3 | ||||||
| Possible (n = 11) | 4 | 7 | – | 5 | 5 | 1 | 5 | 5 | 1 | ||||||
| ‘Not CJD’ (n = 38) | 4 | 32 | 2 | 4 | 34 | – | 7 | 31 | – | ||||||
| Total (n = 193) | 94 | 87 | 12 | 93 | 88 | 12 | 114 | 74 | 5 | ||||||
| . | Observer 1 . | . | . | Observer 2 . | . | . | Observer 3 . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Typical . | Not typical . | Non-diagnostic . | Typical . | Not typical . | Non-diagnostic . | Typical . | Not typical . | Non-diagnostic . | ||||||
| Definite (n = 60) | 39 | 15 | 6 | 34 | 21 | 5 | 38 | 21 | 1 | ||||||
| Probable (n = 84) | 47 | 33 | 4 | 50 | 28 | 6 | 64 | 17 | 3 | ||||||
| Possible (n = 11) | 4 | 7 | – | 5 | 5 | 1 | 5 | 5 | 1 | ||||||
| ‘Not CJD’ (n = 38) | 4 | 32 | 2 | 4 | 34 | – | 7 | 31 | – | ||||||
| Total (n = 193) | 94 | 87 | 12 | 93 | 88 | 12 | 114 | 74 | 5 | ||||||
Inter-observer agreement
In 71 of 193 cases all observers considered the MRIs to be ‘typical for sCJD’. In 52 cases, all observers classified the MRIs to be ‘not typical’. There is thus an overall agreement of 123 of 193 = 63.7% [56.7%; 70.2%]. The overall κ (which incorporates the amount of agreement that is only achieved by chance) is 0.53.
Agreement between Observers 1 and 2 is: κ = 0.55, between Observers 1 and 3: κ = 0.51 and between Observers 2 and 3: κ = 0.55 (Table 2).
Inter-observer agreement
. | Typical . | Not typical . | Non-diagnostic . | Total . | ||||
|---|---|---|---|---|---|---|---|---|
| Observer 1–Observer 2* | ||||||||
| Typical | 76 | 12 | 6 | 94 | ||||
| Not typical | 12 | 69 | 6 | 87 | ||||
| Non-diagnostic | 5 | 7 | 0 | 12 | ||||
| Total | 93 | 88 | 12 | 193 | ||||
| Observer 1–Observer 3† | ||||||||
| Typical | 84 | 8 | 2 | 94 | ||||
| Not typical | 26 | 58 | 3 | 87 | ||||
| Non-diagnostic | 4 | 8 | 0 | 12 | ||||
| Total | 114 | 74 | 5 | 193 | ||||
| Observer 2–Observer 3‡ | ||||||||
| Typical | 85 | 7 | 1 | 93 | ||||
| Not typical | 23 | 61 | 4 | 88 | ||||
| Non-diagnostic | 6 | 6 | 0 | 12 | ||||
| Total | 114 | 74 | 5 | 193 | ||||
. | Typical . | Not typical . | Non-diagnostic . | Total . | ||||
|---|---|---|---|---|---|---|---|---|
| Observer 1–Observer 2* | ||||||||
| Typical | 76 | 12 | 6 | 94 | ||||
| Not typical | 12 | 69 | 6 | 87 | ||||
| Non-diagnostic | 5 | 7 | 0 | 12 | ||||
| Total | 93 | 88 | 12 | 193 | ||||
| Observer 1–Observer 3† | ||||||||
| Typical | 84 | 8 | 2 | 94 | ||||
| Not typical | 26 | 58 | 3 | 87 | ||||
| Non-diagnostic | 4 | 8 | 0 | 12 | ||||
| Total | 114 | 74 | 5 | 193 | ||||
| Observer 2–Observer 3‡ | ||||||||
| Typical | 85 | 7 | 1 | 93 | ||||
| Not typical | 23 | 61 | 4 | 88 | ||||
| Non-diagnostic | 6 | 6 | 0 | 12 | ||||
| Total | 114 | 74 | 5 | 193 | ||||
κ = 0.55;
κ = 0.51;
κ = 0.55.
Inter-observer agreement
. | Typical . | Not typical . | Non-diagnostic . | Total . | ||||
|---|---|---|---|---|---|---|---|---|
| Observer 1–Observer 2* | ||||||||
| Typical | 76 | 12 | 6 | 94 | ||||
| Not typical | 12 | 69 | 6 | 87 | ||||
| Non-diagnostic | 5 | 7 | 0 | 12 | ||||
| Total | 93 | 88 | 12 | 193 | ||||
| Observer 1–Observer 3† | ||||||||
| Typical | 84 | 8 | 2 | 94 | ||||
| Not typical | 26 | 58 | 3 | 87 | ||||
| Non-diagnostic | 4 | 8 | 0 | 12 | ||||
| Total | 114 | 74 | 5 | 193 | ||||
| Observer 2–Observer 3‡ | ||||||||
| Typical | 85 | 7 | 1 | 93 | ||||
| Not typical | 23 | 61 | 4 | 88 | ||||
| Non-diagnostic | 6 | 6 | 0 | 12 | ||||
| Total | 114 | 74 | 5 | 193 | ||||
. | Typical . | Not typical . | Non-diagnostic . | Total . | ||||
|---|---|---|---|---|---|---|---|---|
| Observer 1–Observer 2* | ||||||||
| Typical | 76 | 12 | 6 | 94 | ||||
| Not typical | 12 | 69 | 6 | 87 | ||||
| Non-diagnostic | 5 | 7 | 0 | 12 | ||||
| Total | 93 | 88 | 12 | 193 | ||||
| Observer 1–Observer 3† | ||||||||
| Typical | 84 | 8 | 2 | 94 | ||||
| Not typical | 26 | 58 | 3 | 87 | ||||
| Non-diagnostic | 4 | 8 | 0 | 12 | ||||
| Total | 114 | 74 | 5 | 193 | ||||
| Observer 2–Observer 3‡ | ||||||||
| Typical | 85 | 7 | 1 | 93 | ||||
| Not typical | 23 | 61 | 4 | 88 | ||||
| Non-diagnostic | 6 | 6 | 0 | 12 | ||||
| Total | 114 | 74 | 5 | 193 | ||||
κ = 0.55;
κ = 0.51;
κ = 0.55.
If only the 164 patients whose MRIs were rated either ‘typical’ or ‘not typical’ were included and the 29 patients with ‘non-diagnostic’ exams excluded, the agreement between the observers is even higher; the overall agreement between all three observers improves to 123 of 164 = 75.0% [67.9%; 81.0%]. The overall κ in this case is 0.66. The agreement between Observers 1 and 2 increases to 86% (141 of 164; κ = 0.72), the agreement between Observers 2 and 3 is 82% (135 of 164; κ = 0.64) and the agreement between Observers 1 and 3 is 82% (134 of 164; κ = 0.63).
Field strength
Field strength did not influence the classification of MRIs as either ‘typical’ (0.5 T: 6%; 1 T: 35%; 1.5 T: 55%; not known: 4%) or ‘not typical’ (0.5 T: 6%; 1 T: 44%; 1.5 T: 42%; not known: 8%), nor was there an influence of field strength on the inter-observer agreement or disagreement. The percentage of patients classified uniformly as ‘typical’ or ‘not typical’ was 6% at 0.5 T, 49% at 1 T and 39% at 1.5 T (not known: 6%). There was disagreement in the classification of MRIs as ‘typical’ or ‘not typical’ in 2% of the patients examined at 0.5 T, 39% at 1 T and 54% of those examined at 1.5 T (field strength not known: 5%). In the group of 29 patients classified by at least one of the observers to be ‘non-diagnostic’, there was a higher percentage of patients on whose hard copies the scanner type was not identifiable (24%) than in the group of 164 patients with diagnostic scans (9%) and a higher percentage of 0.5 T exams (14% as compared with 5%).
Sensitivity, specificity and positive predictive value of MRI
The percentage of patients with definite or probable sCJD who showed hyperintense signal changes in MRI was 59.7% [51.6%; 67.4%] (sensitivity with 95% confidence interval) for Observer 1 (86 of 144 cases), 58.3% [50.2%; 66.1%] for Observer 2 (84 of 144 cases) and 70.8% [62.9%; 77.6%] for Observer 3 (102 of 144 cases) (Table 3).
Sensitivity and specificity of MRI analysis
. | Sensitivity [95%-confidence interval] (MRI ‘typical’ in patients with definite or probable sCJD) . | Specificity [95%-confidence interval] (MRI ‘not typical’ in patients clinically or pathologically ‘not-sCJD’) . |
|---|---|---|
| Observer 1 | (86/144) 59.7% [51.6%; 67.4%] | (32/38) 84.2% [69.6%; 92.6%] |
| Observer 2 | (84/144) 58.3% [50.2%; 66.1%] | (34/38) 89.5% [75.9%; 95.8%] |
| Observer 3 | (102/144) 70.8% [62.9%; 77.6%] | (31/38) 81.6% [66.6%; 90.8%] |
. | Sensitivity [95%-confidence interval] (MRI ‘typical’ in patients with definite or probable sCJD) . | Specificity [95%-confidence interval] (MRI ‘not typical’ in patients clinically or pathologically ‘not-sCJD’) . |
|---|---|---|
| Observer 1 | (86/144) 59.7% [51.6%; 67.4%] | (32/38) 84.2% [69.6%; 92.6%] |
| Observer 2 | (84/144) 58.3% [50.2%; 66.1%] | (34/38) 89.5% [75.9%; 95.8%] |
| Observer 3 | (102/144) 70.8% [62.9%; 77.6%] | (31/38) 81.6% [66.6%; 90.8%] |
Sensitivity and specificity of MRI analysis
. | Sensitivity [95%-confidence interval] (MRI ‘typical’ in patients with definite or probable sCJD) . | Specificity [95%-confidence interval] (MRI ‘not typical’ in patients clinically or pathologically ‘not-sCJD’) . |
|---|---|---|
| Observer 1 | (86/144) 59.7% [51.6%; 67.4%] | (32/38) 84.2% [69.6%; 92.6%] |
| Observer 2 | (84/144) 58.3% [50.2%; 66.1%] | (34/38) 89.5% [75.9%; 95.8%] |
| Observer 3 | (102/144) 70.8% [62.9%; 77.6%] | (31/38) 81.6% [66.6%; 90.8%] |
. | Sensitivity [95%-confidence interval] (MRI ‘typical’ in patients with definite or probable sCJD) . | Specificity [95%-confidence interval] (MRI ‘not typical’ in patients clinically or pathologically ‘not-sCJD’) . |
|---|---|---|
| Observer 1 | (86/144) 59.7% [51.6%; 67.4%] | (32/38) 84.2% [69.6%; 92.6%] |
| Observer 2 | (84/144) 58.3% [50.2%; 66.1%] | (34/38) 89.5% [75.9%; 95.8%] |
| Observer 3 | (102/144) 70.8% [62.9%; 77.6%] | (31/38) 81.6% [66.6%; 90.8%] |
Observer 1 considered 32 of 38 ‘not sCJD’ cases not to have hyperintense signal changes in the basal ganglia (specificity 84.2% [70%; 93%]); Observer 2 did not see ‘sCJD typical’ signal changes in 34 of 38 ‘not sCJD’ cases (specificity 89.5% [76%; 96%]) and for observer 3 specificity was 81.6% [67%; 91%] (31 of 38 true negative cases).
The positive predictive value for the MRI diagnosis in sCJD was 95.6% [89.1%; 98.3%] for Observer 1 (86 definite or probable sCJD patients out of 90 patients with positive MRI with 95% confidence interval); 95.5% [88.9%; 98.2%] for Observer 2 (84 of 88 cases) and 93.6% [87.3%; 96.9%] for Observer 3 (102 of 109 cases).
‘Typical’ MRI in ‘not CJD’ cases
MRI was erroneously considered as positive in 4, 4 and 7 (Observers 1, 2 and 3, respectively) of 38 patients who were autopsy-proven (n = 1) or clinically classified (n = 6) ‘not CJD’ cases (see Table 4 for details). One of these ‘MRI false positive’ patients had pathologically confirmed Alzheimer's disease (AD). In this patient, two observers considered the MRI to be ‘typical’ and one observer to be ‘not typical’. This patient's EEG did not show periodic sharp and slow wave complexes, but testing for 14-3-3 protein was positive. The other six patients did not have an autopsy or brain biopsy. One of these patients met laboratory exclusion criteria for sCJD as he had a pleocytosis in CSF. The MRIs of this patient were judged ‘typical’ by two observers and ‘not typical’ by one observer. 14-3-3 protein was positive and EEG showed changes typical for sCJD. The remaining five patients met clinical exclusion criteria for sCJD (disease duration >2 years in two cases) or did not fulfil clinical criteria for sCJD (three cases). These patients either died without necropsy or they were lost in follow-up. In three cases with not enough clinical signs for sCJD, all three observers agreed that the MRI was ‘typical for sCJD’ (including two patients with DW scans). In addition, all these three patients had a positive testing for 14-3-3 protein. In the two remaining cases, one observer considered the MRIs as ‘typical’, the other two observers as ‘not typical’. One of these patients had long disease duration, clinically resembling AD (EEG was not available and 14-3-3 was negative). The other patient did not fulfil clinical criteria, there was only scarce clinical information, and 14-3-3 and EEG were negative.
‘Typical’ MRIs in ‘not sCJD’ patients (n = 7)
| MRI rating (Observers 1/2/3) . | 14-3-3 in CSF . | PSWCs in EEG . | Medical history . |
|---|---|---|---|
| +/−/+* | Positive | Negative | Alzheimer's disease (autopsy-proven) |
| −/+/+ (Including DW images) | Positive | Positive | Pleocytosis in CSF |
| +/+/+ (Including DW images) | Positive | Not available | Disease duration >2 years, died without autopsy |
| +/+/+ (Including DW images) | Positive | Negative | Clinical criteria not fulfilled, died without autopsy |
| +/+/+ | Positive | Negative | Clinical criteria not fulfilled, lost in follow-up |
| −/−/+ | Negative | Negative | Disease duration >2 years, lost in follow-up |
| −/−/+ | Negative | Negative | Clinical criteria not fulfilled, lost in follow-up |
| MRI rating (Observers 1/2/3) . | 14-3-3 in CSF . | PSWCs in EEG . | Medical history . |
|---|---|---|---|
| +/−/+* | Positive | Negative | Alzheimer's disease (autopsy-proven) |
| −/+/+ (Including DW images) | Positive | Positive | Pleocytosis in CSF |
| +/+/+ (Including DW images) | Positive | Not available | Disease duration >2 years, died without autopsy |
| +/+/+ (Including DW images) | Positive | Negative | Clinical criteria not fulfilled, died without autopsy |
| +/+/+ | Positive | Negative | Clinical criteria not fulfilled, lost in follow-up |
| −/−/+ | Negative | Negative | Disease duration >2 years, lost in follow-up |
| −/−/+ | Negative | Negative | Clinical criteria not fulfilled, lost in follow-up |
−, MRI ‘not typical’ for sCJD; +, MRI ‘typical’ for sCJD.
‘Typical’ MRIs in ‘not sCJD’ patients (n = 7)
| MRI rating (Observers 1/2/3) . | 14-3-3 in CSF . | PSWCs in EEG . | Medical history . |
|---|---|---|---|
| +/−/+* | Positive | Negative | Alzheimer's disease (autopsy-proven) |
| −/+/+ (Including DW images) | Positive | Positive | Pleocytosis in CSF |
| +/+/+ (Including DW images) | Positive | Not available | Disease duration >2 years, died without autopsy |
| +/+/+ (Including DW images) | Positive | Negative | Clinical criteria not fulfilled, died without autopsy |
| +/+/+ | Positive | Negative | Clinical criteria not fulfilled, lost in follow-up |
| −/−/+ | Negative | Negative | Disease duration >2 years, lost in follow-up |
| −/−/+ | Negative | Negative | Clinical criteria not fulfilled, lost in follow-up |
| MRI rating (Observers 1/2/3) . | 14-3-3 in CSF . | PSWCs in EEG . | Medical history . |
|---|---|---|---|
| +/−/+* | Positive | Negative | Alzheimer's disease (autopsy-proven) |
| −/+/+ (Including DW images) | Positive | Positive | Pleocytosis in CSF |
| +/+/+ (Including DW images) | Positive | Not available | Disease duration >2 years, died without autopsy |
| +/+/+ (Including DW images) | Positive | Negative | Clinical criteria not fulfilled, died without autopsy |
| +/+/+ | Positive | Negative | Clinical criteria not fulfilled, lost in follow-up |
| −/−/+ | Negative | Negative | Disease duration >2 years, lost in follow-up |
| −/−/+ | Negative | Negative | Clinical criteria not fulfilled, lost in follow-up |
−, MRI ‘not typical’ for sCJD; +, MRI ‘typical’ for sCJD.
Comparison of MR sequences
MR sequences were compared for the patients who were classified to have ‘typical’ MRI by at least one of the observers and whose final pathological or clinical diagnosis was definite or probable sCJD. As each of the observers analysed the MR scans independently, the number of patients with ‘typical’ MRIs in this group of patients was different depending on the observer. Comparison of sequences was done for only those patients who provided at least two MR sequences.
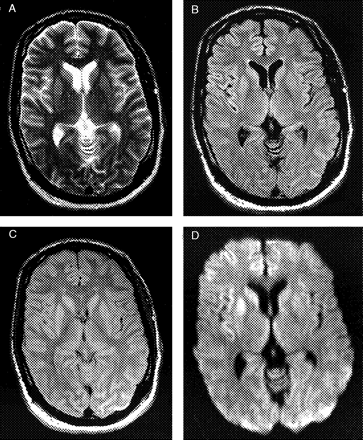
The T2-weighted sequence was considered to show the pathologic changes best in 9 of 72, 8 of 66 and 19 of 82 cases (Observers 1, 2 and 3, respectively). This is an overall percentage of 16.3. FLAIR images were rated to best show the signal changes in 31 of 55, 28 of 52 and 20 of 60 ‘typical’ cases, which is an overall percentage of 47.3. The PD weighted sequence was graded to be the best in 8 of 22, 5 of 17 and 10 of 30 ‘typical’ sCJD patients, which is an overall percentage of 33.3. For the DW sequence the rating was best sequence in 27 of 36, 20 of 26 and 38 of 46 cases. This corresponds to an overall 76.6% (Fig. 1A–D).
T2-Weighted (A), FLAIR (B), PD-Weighted (C) and DW (D) sequences of a patient with autopsy-proven sCJD. The MRI was rated as ‘typical for sCJD’ by all three observers. Scans show high signal in the caudate nucleus and putamen bilaterally. In the DW sequence (D) a predominance of the right striatum and additionally increased signal in the right frontal and insular cortex is discernible.
Comparing only those patients in whom all four sequences were available, the DW sequences were rated as the best in 7 of 8, 5 of 7 and 9 of 10 patients (Observers 1, 2 and 3, respectively).
Sensitivity and specificity of EEG and 14-3-3
The test for 14-3-3 protein in CSF had a sensitivity of 91% and a specificity of 44%.
Sensitivity of EEG was 32% and specificity was 94% (Table 5).
Comparison of 14-3-3, EEG and MRI
. | 14-3-3 in CSF: current study . | 14-3-3 in CSF: literature* . | PSWCs in EEG: current study . | PSWCs in EEG: literature** . | Hyperintense grey matter in MRI: current study . | Hyper intense grey matter in MRI: literature** . |
|---|---|---|---|---|---|---|
| sCJD (definite/probable) | 128/140 | 205/219 | 42/133 | 144/219 | 86/144 (Obs. 1) 84/144 (Obs. 2) 102/144 (Obs. 3) | 109/162 |
| Not sCJD (clinically/pathologically) | 19/34 | 7/43 | 2/32 | 11/43 | 4/38 (Obs. 1) 4/38 (Obs. 2) 7/38 (Obs. 3) | 4/58 |
| Sensitivity (%) | 91 | 94 | 32 | 66 | 60 (Obs. 1) 58 (Obs. 2) 71 (Obs. 3) | 67 |
| Specificity (%) | 44 | 84 | 94 | 74 | 84 (Obs. 1) 90 (Obs. 2) 82 (Obs. 3) | 93 |
. | 14-3-3 in CSF: current study . | 14-3-3 in CSF: literature* . | PSWCs in EEG: current study . | PSWCs in EEG: literature** . | Hyperintense grey matter in MRI: current study . | Hyper intense grey matter in MRI: literature** . |
|---|---|---|---|---|---|---|
| sCJD (definite/probable) | 128/140 | 205/219 | 42/133 | 144/219 | 86/144 (Obs. 1) 84/144 (Obs. 2) 102/144 (Obs. 3) | 109/162 |
| Not sCJD (clinically/pathologically) | 19/34 | 7/43 | 2/32 | 11/43 | 4/38 (Obs. 1) 4/38 (Obs. 2) 7/38 (Obs. 3) | 4/58 |
| Sensitivity (%) | 91 | 94 | 32 | 66 | 60 (Obs. 1) 58 (Obs. 2) 71 (Obs. 3) | 67 |
| Specificity (%) | 44 | 84 | 94 | 74 | 84 (Obs. 1) 90 (Obs. 2) 82 (Obs. 3) | 93 |
Comparison of 14-3-3, EEG and MRI
. | 14-3-3 in CSF: current study . | 14-3-3 in CSF: literature* . | PSWCs in EEG: current study . | PSWCs in EEG: literature** . | Hyperintense grey matter in MRI: current study . | Hyper intense grey matter in MRI: literature** . |
|---|---|---|---|---|---|---|
| sCJD (definite/probable) | 128/140 | 205/219 | 42/133 | 144/219 | 86/144 (Obs. 1) 84/144 (Obs. 2) 102/144 (Obs. 3) | 109/162 |
| Not sCJD (clinically/pathologically) | 19/34 | 7/43 | 2/32 | 11/43 | 4/38 (Obs. 1) 4/38 (Obs. 2) 7/38 (Obs. 3) | 4/58 |
| Sensitivity (%) | 91 | 94 | 32 | 66 | 60 (Obs. 1) 58 (Obs. 2) 71 (Obs. 3) | 67 |
| Specificity (%) | 44 | 84 | 94 | 74 | 84 (Obs. 1) 90 (Obs. 2) 82 (Obs. 3) | 93 |
. | 14-3-3 in CSF: current study . | 14-3-3 in CSF: literature* . | PSWCs in EEG: current study . | PSWCs in EEG: literature** . | Hyperintense grey matter in MRI: current study . | Hyper intense grey matter in MRI: literature** . |
|---|---|---|---|---|---|---|
| sCJD (definite/probable) | 128/140 | 205/219 | 42/133 | 144/219 | 86/144 (Obs. 1) 84/144 (Obs. 2) 102/144 (Obs. 3) | 109/162 |
| Not sCJD (clinically/pathologically) | 19/34 | 7/43 | 2/32 | 11/43 | 4/38 (Obs. 1) 4/38 (Obs. 2) 7/38 (Obs. 3) | 4/58 |
| Sensitivity (%) | 91 | 94 | 32 | 66 | 60 (Obs. 1) 58 (Obs. 2) 71 (Obs. 3) | 67 |
| Specificity (%) | 44 | 84 | 94 | 74 | 84 (Obs. 1) 90 (Obs. 2) 82 (Obs. 3) | 93 |
Discussion
The neuroimaging hallmark of sCJD is increased grey matter signal on T2-weighted, FLAIR, PD and DW MRIs. In most cases, MRI shows bilateral symmetric markedly hyperintense caudate nuclei and putamina, whereas the thalami and the cortex are usually involved to a lesser degree. In contrast, vCJD shows increased signal of the pulvinar thalami generally exceeding the signal of the caudate nuclei and putamina (Collie et al., 2003).
Some studies have reported on high sensitivity of MRI to detect typical high signal increase in basal ganglia in sCJD, when analysed by a specialist and in context of diagnosis of sCJD (Finkenstaedt et al., 1996; Schroter et al., 2000; Collie et al., 2001; Urbach et al., 2001). However, these changes were often missed, when the diagnosis of CJD was not considered, as has been shown by an evaluation of radiology reports (Zeidler et al., 1996). The interpretation of signal hyperintensities in the basal ganglia is hard to standardize and might be considered to be observer-dependent to some extent. Up to now, only limited data were available addressing this issue. In our study, we show that the agreement for the MRI interpretation is high (63.7%) between the three observers (overall κ = 0.53). This finding underlines the power of MRI as a potential diagnostic tool for sCJD. The present study is the largest, which deals with the inter-observer agreement in the MR diagnosis of sCJD. In the two previous studies of our group, only one radiologist analysed the MRIs (Finkenstaedt et al., 1996; Schroter et al., 2000). The paper from Zeidler et al. (2000) addressed the issue of subjective reporting and analysed inter-observer and intra-observer agreement in the diagnosis of vCJD. Collie et al. (2003) reported on inter-observer agreement of two observers in a retrospective study on 86 neuropathologically confirmed vCJD patients with kappa values were between 0.64 for PD and 1.0 for FLAIR scans (overall κ = 0.65). A recent work from Shiga et al. (2004) also addressed the issue of inter-observer agreement, comparing two observers who evaluated the MRIs of 36 patients. Depending on the sequence, they reached an inter-observer agreement between 68.2% for T2 weighted and 100% for DW images. One should keep in mind, that in our study three observers were included, that the observers were blinded to clinical data and that we introduced a third category (‘non-diagnostic’) owing to the wide quality range of images provided. Comparing only those images, which were rated by all three observers to be of diagnostic value, inter-observer agreement for the three observers is 75% (overall κ = 0.66). Agreement for only two observers was even higher: 86% for Observers 1 and 2 and 82% for Observers 1 and 3 or Observers 2 and 3.
In the current study, 58% of sCJD cases had typical MRI changes (sensitivity) as rated by three independent observers. This is less than the value previously described by Finkenstaedt (Finkenstaedt et al., 1996) and Schroter (Schroter et al., 2000) who found a sensitivity of 79 and 67%, respectively. With refinement of MRI studies and introduction of the DW sequence one would expect a higher sensitivity in the present study than in former ones. Reasons for the lower sensitivity in the actual study can only be assumed. In our opinion, one reason is the mode of collection of MRI hard copies, which has changed over time. The study of Finkenstaedt et al. (1996) reports on MR exams of 29 CJD patients, most probably collected during the first 2 years of the German CJD Surveillance study (1993–1995), without giving a non-CJD dementia control group. In the study of Schroter et al. (2000), MRIs of 245 patients and controls, collected during the years 1993–1998 were analysed. In the first years of the German CJD Surveillance study (which started in 1993), MRIs of only a small number of patients were collected [245 in 6 years (Schroter et al., 2000) as compared with 193 in 2 years in our present study]. We believe that in the former studies there was a preselection of MRI exams of good quality or with typical findings. Since the molecular basis for different disease phenotypes in sCJD was established (Parchi et al., 1999), it has been shown that sensitivity of clinical tests varies among phenotypes (Zerr et al., 2000b). As a consequence, all MRI scans were collected in all suspected CJD patients in recent years in order to avoid an ascertainment bias. In addition, since MRI is increasingly recognized as a potential diagnostic technique in CJD, more scans are performed at possibly earlier disease stages, which might also alter the test sensitivity. Considering the latter hypothesis, sequential MRI scans should be performed in clinically atypical cases, especially if 14-3-3 protein and EEG were negative.
It has to be kept in mind that the high sensitivity values in the studies mentioned above are because of the fact that they were performed in a reference centre for CJD. Thus, the observers were aware of CJD as a differential diagnosis. Schroter et al. (2000) reported that in the centre of first attendance the radiologists or neuroradiologists overlooked the MRI changes in 80% of cases, as they are symmetrical and sCJD is a very rare disease. This interpretation is in line with an earlier observation (Zeidler et al., 1996). In the latter study, hyperintense basal ganglia were reported in 4% by local radiologists. In our study, only in 28% MR-high signal in the basal ganglia was described in the centre of first attendance. As the correct MRI reading in the differential diagnosis of CJD needs specialist experience and for surveillance purposes, we strongly recommend that the interpretation of the MRI scans should be done in a specialized centre.
The gold standard for the evaluation of a diagnostic test in sCJD is the definite diagnosis given by autopsy. As it was not possible to have a necropsy of all studied patients, the current clinical diagnostic criteria were used to evaluate the accuracy of the MRI as an additional diagnostic test. According to our data, the overall sensitivity of the MRI was at least 58% and the specificity was minimum 82%. We analysed the reasons for ‘false-positive’ MRI tests in the group of patients who were diagnosed as ‘non-CJD’ cases.
Depending on the observer, MRI was erroneously considered as positive in 4, 4 and 7 of 38 patients who were classified as ‘non-CJD’ cases (specificity 84.2, 89.5 and 81.6%, Observers 1–3).
We cannot rule out that some of the clinically ‘non-sCJD’ patients with ‘false positive MRI’ actually had sCJD, not identified by the current clinical diagnostic criteria (Table 4) (Poser et al., 1999). Positive 14-3-3 test in CSF was present in five of these seven ‘false positive MRI’ cases. EEG showed PSWCs in one of six patients. There was one necropsy-proven AD patient among the ‘false positive’ MRI cases and another patient with pleocytosis in the CSF, which is an exclusion criteria for sCJD. The five other cases were defined by clinical criteria not to have sCJD. Among these, three patients were considered by all three observers of this study to have a ‘sCJD typical’ MRI, including DW scans in two cases. Unfortunately, since these cases were lost in the follow-up or died without autopsy we will not be able to definitely resolve the question whether they had sCJD (not covered by current clinical diagnostic criterion) or not. One can speculate that these patients were notified to the CJD Surveillance Unit at an early disease stage and might meet CJD criteria after a follow-up. Unfortunately, no further information on these patients is available.
Overlap of the MRI changes with other conditions, such as encephalitis and hypoxia, CO poisoning, hypoglycaemia, Wilson's disease, mitochondrial diseases (e.g. Leigh's disease) and Huntington's disease, are described. Usually, these conditions can clinically be clearly distinguished. Except for encephalitis, they were not in the differential diagnosis in our patients with ‘false positive’ MRI. Specificity in the present study was in accordance with the reported 93% from Schroter et al. (2000).
Until now, the question, which MRI sequences are the most sensitive to display hyperintense basal ganglia, is not solved. Increasing evidence points that DW images might be the best to detect these abnormalities in CJD (Bahn et al., 1997; Bahn and Parchi, 1999; Kropp et al., 2000; Sellars et al., 2002; Tschampa et al., 2003; Shiga et al., 2004). Our data are in line with these reports. Comparing the diagnostic utility of the sequences, the DW images were unanimously considered to best show the signal changes in 76.6% of definite or clinically probable cases, followed by the FLAIR sequence (47%). In a direct comparison of the four sequences (T2-W, FLAIR, PD-W, DW) again the DW sequence was rated to give the best diagnostic information. From the technical point of view, DW imaging is advantageous in the evaluation of a suspect CJD patient: DW sequences are short, limiting the risk of motion artefacts. FLAIR sequences were the second most sensitive for the detection of CJD typical signal changes; in addition, they give more structural information than DW scans and are helpful in the detection of other potential causes of the clinical symptoms (such as encephalitis or tumour). We thus recommend the inclusion of DW and FLAIR sequences in an optimal imaging protocol for suspected sCJD patients. As most knowledge about MRI changes in sCJD patients is based on T2-weighted studies, this sequence could be included in the imaging protocol as an option.
The EEG and 14-3-3 results in our study differed from data reported earlier (Zerr et al., 2000a, b). Sensitivity of EEG in the current study with 32% was lower and specificity was higher than reported previously (Steinhoff et al., 1996; Zerr et al., 2000a). During the last years of the Surveillance Study, the referral of patients to the Surveillance Unit was based on the clinical signs and CSF, and to a lesser extent on the EEG. It is likely that cases were referred at an earlier stage, when the EEG does not yet show typical PSWCs (I. Zerr, personal communication).
Sensitivity of 14-3-3 protein analysis (91%) was comparable with earlier studies (94% by Zerr et al., 2000a) whereas the specificity (44%) was lower than expected (84% by Zerr et al., 2000a). This is most probably because of a recent change in case referral. During the time of recruitment of the patients for our study (2001–2003) clinicians were aware of the high sensitivity of the 14-3-3 testing. Thus, 14-3-3 protein analysis was used as a ‘screening test’ for suspected sCJD patients; and clinical examination by a member of the CJD Surveillance Unit was often performed after 14-3-3 protein test in CSF. As a consequence of this inappropriate use of the 14-3-3 analysis, more patients with elevated 14-3-3 protein levels in CSF owing to other acute neurological illnesses (e.g. encephalitis, stroke, epileptic fits) were included in the study. This led to a higher rate of false positive 14-3-3 cases and thus, a lower specificity of 14-3-3.
Conclusion
We conclude that MRI is a reliable non-invasive diagnostic test for sCJD. We suggest that MRI workup of suspected sCJD patients should include DW and FLAIR scans, and we propose to incorporate MRI in the diagnostic criteria for sCJD, in addition to EEG and 14-3-3 protein analysis.
This study was supported by a grant from the Bundesministerium für Gesundheit, Bonn, Germany, to H.A.K. and Sigrid Poser (BMG Gz 325-4471-02/15). The authors thank all physicians who referred suspect patients to the German CJD Surveillance unit for providing clinical data, MRIs and necropsy brains. We would also like to express our thanks to the physicians of the CJD Surveillance Unit for clinical evaluation of suspected CJD patients. We thank Bernhard J. Steinhoff and Sigrid Poser for EEG-analyses, Monika Bodemer for 14-3-3 analyses and Maja Schneider-Dominco for her technical assistance.
References
Bahn MM, Kido DK, Lin W, Pearlman AL. Brain magnetic resonance diffusion abnormalities in Creutzfeldt-Jakob disease.
Bahn MM, Parchi P. Abnormal diffusion-weighted magnetic resonance images in Creutzfeldt-Jakob disease.
Collie DA, Sellar RJ, Zeidler M, Colchester AC, Knight R, Will RG. MRI of Creutzfeldt-Jakob disease: imaging features and recommended MRI protocol.
Collie DA, Summers DM, Sellar RJ, Ironside JW, Copper S, Zeidler M, et al. Diagnosing variant Creutzfeldt-Jakob disease with the pulvinar sign: MR imaging findings in 86 neuropathologically confirmed cases.
Finkenstaedt M, Szudra A, Zerr I, Poser S, Hise JH, Stoebner JM, et al. MR imaging of Creutzfeldt-Jakob disease.
Kretzschmar HA, Ironside JW, DeArmond SJ, Tateishi J. Diagnostic criteria for sporadic Creutzfeldt-Jakob disease.
Kropp S, Finkenstaedt M, Zerr I, Schroter A, Poser S. Diffusion-weighted MRI in patients with Creutzfeldt-Jakob disease.
Masters CL, Harris JO, Gajdusek DC, Gibbs CJ Jr, Bernoulli C, Asher DM. Creutzfeldt-Jakob disease: patterns of worldwide occurrence and the significance of familial and sporadic clustering.
Newcombe R, Altman D. Proportions and their differences. In: Altman D, Machin D, Bryant T, Gardner M, editors. Statistics with confidence. British Medical Journal Books, Bristol,
Parchi P, Giese A, Capellari S, Brown P, Schulz-Schaeffer W, Windi O, et al. Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects.
Poser S, Mollenhauer B, Kraubeta A, Zerr I, Steinhoff BJ, Schroeter A, et al. How to improve the clinical diagnosis of Creutzfeldt-Jakob disease.
Schroter A, Zerr I, Henkel K, Tschampa HJ, Finkenstaedt M, Poser S. Magnetic resonance imaging in the clinical diagnosis of Creutzfeldt-Jakob disease.
Sellars RJ, Collie DA, Will RJ. Progress in understanding Creutzfeldt-Jakob disease.
Shiga Y, Miyazawa K, Sato S, Fukushima R, Shibuya S, Sato Y, et al. Diffusion-weighted MRI abnormalities as an early diagnostic marker for Creutzfeldt-Jakob disease.
Steinhoff BJ, Racker S, Herrendorf G, Poser S, Grosche S, Zerr I, et al. Accuracy and reliability of periodic sharp wave complexes in Creutzfeldt-Jakob disease.
Tschampa HJ, Murtz P, Flacke S, Paus S, Schild HH, Urbach H. Thalamic involvement in sporadic Creutzfeldt-Jakob disease: a diffusion-weighted MR imaging study.
Urbach H, Klisch J, Wolf HK, Brechtelsbauer D, Gass S, Solymosi L. MRI in sporadic Creutzfeldt-Jakob disease: correlation with clinical and neuropathological data.
Urbach H, Paus S, Tschampa HJ, Keller E, Schild HH. Creutzfeldt-Jakob disease: value of MRI.
Will RG, Zeidler M, Stewart GE, Macleod MA, Ironside JW, Cousens SN, et al. Diagnosis of new variant Creutzfeldt-Jakob disease.
World Health Organization. Human transmissible spongiform encephalopathies. Weekly Epidemiological Record
Zeidler M, Will RG, Ironside JW, Sellar R, Wardlaw J. Magnetic resonance imaging is not a sensitive test for Creutzfeldt-Jakob disease.
Zeidler M, Sellar RJ, Collie DA, Knight R, Stewart G, Macleod MA, et al. The pulvinar sign on magnetic resonance imaging in variant Creutzfeldt-Jakob disease.
Zerr I, Bodemer M, Gefeller O, Otto M, Poser S, Wiltfang J, et al. Detection of 14-3-3 protein in the cerebrospinal fluid supports the diagnosis of Creutzfeldt-Jakob disease.
Zerr I, Pocchiari M, Collins S, Brandel JP, de Pedro CJ, Knight RS, et al. Analysis of EEG and CSF 14-3-3 proteins as aids to the diagnosis of Creutzfeldt-Jakob disease.
Author notes
1Department of Radiology and 2Institute for Medical Biometry, Informatics and Epidemiology, University of Bonn, 3Department of Neuroradiology and 4Department of Neurology, Georg-August University, Göttingen and 5Institute for Neuropathology, Ludwig-Maximilians University, München, Germany