Published online Oct 28, 2015. doi: 10.4329/wjr.v7.i10.329
Peer-review started: May 11, 2015
First decision: June 24, 2015
Revised: July 31, 2015
Accepted: August 20, 2015
Article in press: August 21, 2015
Published online: October 28, 2015
Malformation of cortical development (MCD) is a term representing an inhomogeneous group of central nervous system abnormalities, referring particularly to embriyological aspect as a consequence of any of the three developmental stages, i.e., cell proliferation, cell migration and cortical organization. These include cotical dysgenesis, microcephaly, polymicrogyria, schizencephaly, lissencephaly, hemimegalencephaly, heterotopia and focal cortical dysplasia. Since magnetic resonance imaging is the modality of choice that best identifies the structural anomalies of the brain cortex, we aimed to provide a mini review of MCD by using 3T magnetic resonance scanner images.
Core tip: Malformations of cortical development reflect embryological disruptions in neuronal proliferation, neuronal migration, cortical organization or multiple steps of the cortical development. Malformation of cortical development (MCDs) are important causes of refractory epilepsy. Magnetic resonance imaging including the advanced MRI techniques has a pivotal role in diagnosis of MCDs and prediction of feasibility of surgical management in cases that are refractory to medication.
- Citation: Battal B, Ince S, Akgun V, Kocaoglu M, Ozcan E, Tasar M. Malformations of cortical development: 3T magnetic resonance imaging features. World J Radiol 2015; 7(10): 329-335
- URL: https://www.wjgnet.com/1949-8470/full/v7/i10/329.htm
- DOI: https://dx.doi.org/10.4329/wjr.v7.i10.329
Malformations of cortical development (MCDs) are a complex group of abnormalities that may occur due to interruption the stages of proliferation, migration or postmigrational development of cortex. MCDs are common and important cause of seizures particularly in children. MCDs may result from lack of migration, over migration or ectopic migration of immature neurons[1]. Magnetic resonance imaging (MRI) is the modality of choice that allows diagnosis of MCDs and correlation between radiologic and clinicopathologic findings related to MCDs[2]. New techniques such as functional MRI (fMRI), MR spectroscopy and diffusion tensor imaging (DTI) are useful particularly for the detection of small sized or subtle MCDs[2]. Barkovich et al[3] described the latest updated version of classification scheme for MCDs in 2012.
In this mini-review article, we discuss different types of cortical malformations in terms of their MRI characteristics. We illustrate their imaging features with emphasis on lesion location and direction of extension.
MRI is the preferred imaging technique for determination and detailed assessment of MCD, with detailed information available from ultimate MR sequences and modalities. Advanced MRI sequences and techniques including volumetric three-dimensional (3D) gradient recalled echo (GRE) T1-weighted sequences, volumetric fluid attenuated inversion recovery (FLAIR), susceptibility weighted imaging (SWI), MR spectroscopy, fMRI and DTI have improved image quality and have been helpful in detection and characterization of MCDs and detailed evaluation of its extent and relationship with the surrounding white and gray matter areas.
Volumetric 3D GRE T1-weighted sequences allow high resolution multiplanar reformatted images, and support accurate diagnosis and detailed evaluation. For this purpose, slices of 1 mm thickness are gained with isotropic voxels. This allows high spatial resolution, more detailed evaluation of cortical thickness and morphology, and gray-white matter differentiation in three orthogonal planes in a reasonable time frame for clinical use. Volumetric FLAIR sequence may be useful in the determination of small signal changes and correct delineation of the borders of MCD and related signal changes, especially in 3T. SWI is a new, full-velocity-compensated high-resolution 3D GE sequence, useful for the evaluation of various pathologies characterized with punctate hemorrhages and calcification in brain parenchyma[4,5].
Metabolic components of the brain tissue can be determined by proton MR spectroscopy in vivo. Simister et al[6] reported that large cortical malformations had abnormal levels of both glutamate + glutamine and gamma-aminobutyric acid. Low N-acetylaspartate (NAA) and high choline (Cho) levels were also observed. They concluded that MCD showed spectroscopic features of primitive tissue and abnormal metabolisms of both inhibitory and excitatory neurotransmitters. MR spectroscopy technique demonstrated that MCD extends beyond the borders determined by the conventional MR imaging, even with sophisticated volumetric techniques[7,8]. In a phosphorus MR spectroscopic study, Andrade et al[9] demonstrated widespread acidosis in the normal appearing parenchyma, and they concluded that visible lesions of the MCD are only the tip of the iceberg. MR spectroscopy examination can also be useful in differentiating low-grade tumor from focal cortical dysplasia (FCD) based on imaging can be challenging but important for treatment planning. The main difference was low level of NAA in gliomas compared to nearly normal levels of NAA in FCD and dysembryoplastic neuroectodermal tumor (DNET). In contrast, there were increased Cho levels in low-grade glioma compared to FCD.
fMRI can localize brain functions based on changes in blood flow and its data can be used in pre-surgical mapping. For the functional evaluation of the MCD, simple (sensomotor, visual) or complex (language, memory) fMRI paradigms can be used. fMRI technique can visualize the functions of the MCDs and their relationship to the eloquent cortex, and can provide important information to surgeons prior to epilepsy surgery[10].
DTI gives microstructural data about tissues and provides further information on brain tissue that cannot be obtained by using conventional MR sequences. Microstructure of the tissue may change due to increase or decrease of neuronal cell volume, enlarged or reduced extracellular space and tissue damage, thus altered diffusion and fractional anisotropy may occur[11]. MCD have features of loss of tissue organization and abnormalities of neuronal structure, but preserved normal cellular density. Therefore, reduced anisotropy but normal diffusivity values similar to the normal tissue are characteristic DTI findings of the MCD[12,13].
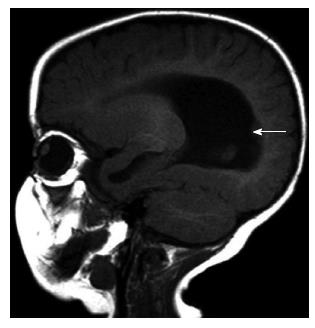
Cortical dysgenesis: Hemimegalencephaly is determined as a cortical dysgenesis in this new classification[3]. Hemimegalencephaly is a hamartomatous malformation characterized by overgrowth of all or part of one cerebral hemisphere. Three different types are described. First type is isolated hemimegalencephaly, which is no hemicorporal hypertrophy and not associated with cutaneous or systemic involvement (Figure 1). Second type is hemimegalencephaly associated with various neurocutaneous syndromes such as neurofibromatosis type 1, epidermal nevus syndrome, proteus syndrome, and tuberous sclerosis and also typically including hemicorporal hypertrophy of the ipsilateral part of the body. The last and the rarest one is total hemimegalencephaly associated with hypertrophy of the ipsilateral cerebellar hemisphere and brainstem. The cerebral cortical structures may be normal or dysplastic[14,15].
Unlike other types of FCDs, FCD type II (FCD-II) is accepted in-group of cortical dysgenesis in the new classification of Barkovich et al[3] FCD-II has divided into two subgroups as type a and b[16]. FCD-II is characterized by focal area of cortical thickening, marked blurring of gray and white matter junction, and in some cases signal change in adjacent white matter on T2-weighted and FLAIR images[17]. On the other hand, it is harder to identify FCD-IIa than FCD-IIb, and FCD-IIa cannot be always detected on MRI[16].
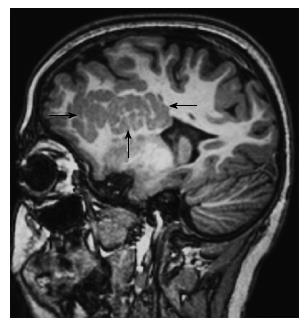
FCDs can mimic gliomas. FCD-II often occurs in extra-temporal location especially frontal region, whereas a temporal location suggests gliomas[18]. Since the cortical dysplasia primarily affect the gray matter and generally not associated with edema and gliosis, the increased signal on T2-weighted images is less distinct in FCDs when compared with the tumors (Figure 2). Gliomas may cause mass effect and frequently show intravenous contrast enhancement[1].
Microcephaly: Congenital microcephaly refers to a head circumference of patient < 3 standard deviation below normal for that age without history of intrauterine damage[1]. There is also another microcephaly entity, which is classified in abnormal postmigrational development group. This type patients born with normal or small head size, and severe microcephaly progresses within 1-2 years after the birth[3].
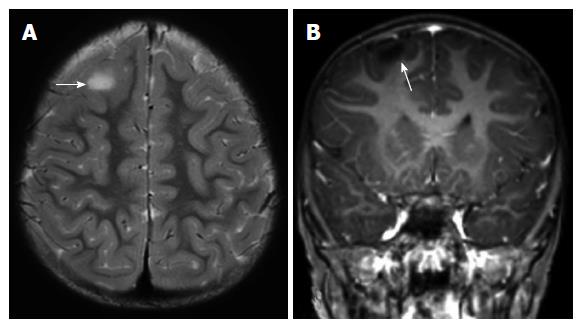
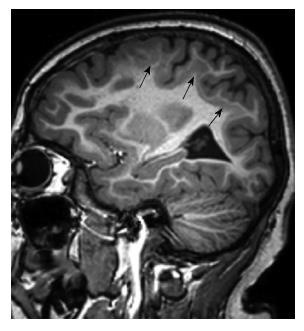
Microcephaly with a simplified gyral pattern is a mild form of microlissencephaly, and characterized with profound microcephaly, significantly decreased number of sulci, sulcation abnormality, but normal cortical thickness (Figure 3). This malformation is generally not associated with other congenital structural anomalies[1].
Periventricular heterotopia (subependymal nodular): Heterotopia is an abnormal location of neurons anyplace between the subependymal region of the lateral ventricles and cerebral cortex secondary to arrest of radial migration[1].
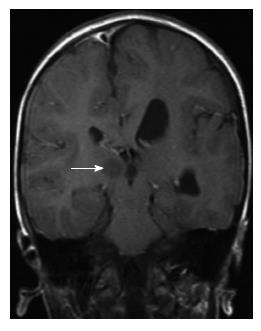
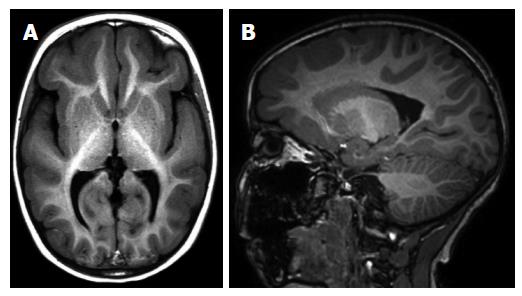
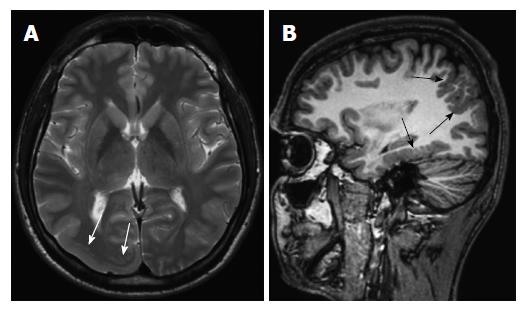
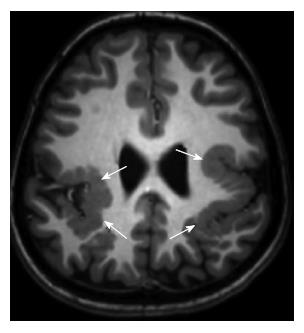
Periventricular (subependymal nodular) heterotopias are often bilateral and placed adjacent to the walls of the lateral ventricles, frequently in the peritrigonal regions, the temporal and occipital horns[1]. The characteristic appearances of nodular heterotopia are small, round or oval shaped nodules isointense with gray matter on all MR sequences, located in subependymal layer, and do not show contrast enhancement (Figure 4). Periventricular heterotopia may project into the ventricular lumen (Figure 5)[1].
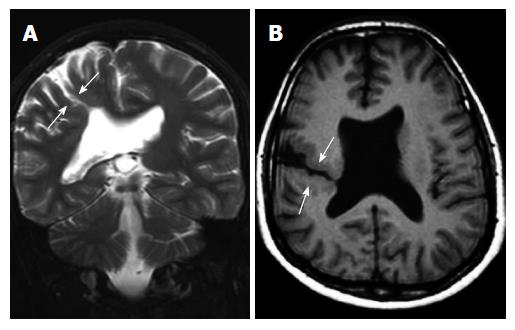
Subcortical heterotopia (Band heterotopia): Subcortical band heterotopias (SCH) are located within the subcortical or deep white matter between the ventricular epandimal surface and the cerebral cortex. SCH may be in various forms including nodular, curvilinear or mixed. Thin overlying cortex and shallow sulci are characteristic findings for SCH (Figure 6)[1,19].
SCH is a rare developmental malformation seen primarily in female and may be familial with X-linked dominant inheritance. On MRI, it shows a characteristic two parallel layers of gray matter separated by a thin white matter layer (3-layer cake: A thin outer ribbon, a very thin layer of white matter and a thick inner band (Figure 7)[1,19].
Lissencephaly: Lissencephaly is defined as smooth brain and characterized with the absent normal gyral and sulcal pattern of the brain. The neocortex of lissencephalic patients lacks the normal cortical lamination and contains four layers instead of the six[19]. Classic or type 1 lissencephaly appears as a complete or incomplete agyria. The complete form is characterized with smooth surface of entire brain. In the incomplete form, which is more commonly seen type, temporal lobes and inferior regions of frontal lobes have some gyral formation (Figure 8). The important MRI findings of classical lissencephaly are hourglass configuration, thick cortex and thin subcortical white matter, pachygyria-agyria areas, lack of gray-white matter interdigitation, and shallow Sylvian fissure[1,19].
Cobblestone malformation: Cobblestone lissencephaly or formerly called type 2 lissencephaly is characterized by a nodular cortical surface accompanied by ocular anomalies and congenital muscular disorders[1]. Cobblestone lissencephaly has been divided into three different groups based on severity: (1) Cobblestone lissencephaly occurs in various genetic conditions, with Walker-Warburg syndrome being the most severe form; (2) Fukuyama congenital muscular dystrophy, the mildest form; and (3) muscle-eye brain disease, the moderate form. Congenital muscular dystrophy is a key feature (Figure 9)[19].
Polymicrogyria: Polymicrogyria (PMG) is caused by an interruption in normal cerebral cortical development in the late neuronal migration or early postmigrational development periods[20]. The cortical surface appears thick and irregular or can have multiple small gyri separated by shallow sulci.
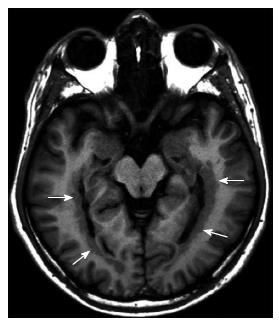
In the cases of PMG, the cerebral cortex may be affected various degrees: Unilateral or bilateral; asymmetrical or symmetrical; single focal focus, multifocal, or diffuse. Around the Sylvian fissure, mostly posterior perisylvian area is the most common location (Figure 10)[21].
For identifying PMG the combination of three characteristics on MR images has been used: (1) abnormal gyral pattern; (2) increased cortical thickness; and (3) irregularity of the cortical–white matter junction due to packing of microgyri[22]. These can be readily detected when thin sliced volumetric images are obtained[21].
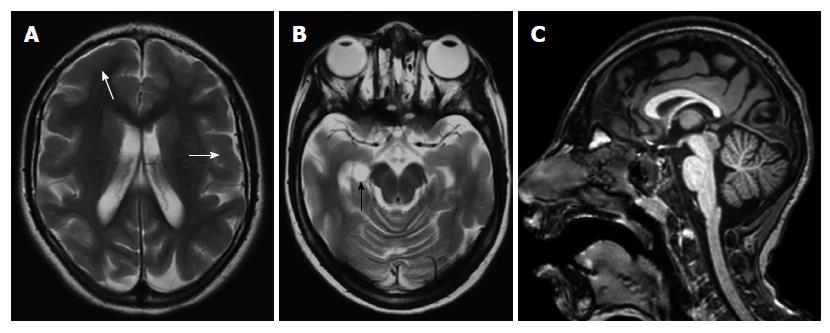
Schizencephaly: Schizencephaly is characterized by unilateral or bilateral full thickness cleft that is extending from the subarachnoid spaces to the ventricular system, and lined with gray matter[23]. The cleft is often found in perisylvian areas, and may be small (closed lip type) or large (open lip type)[24]. It may be associated with other malformations such as agenesis or dysgenesis of optic nerves, septum pellucidum (Figure 11), corpus callosum or hypocampus[25].
FCD: FCD type I (FCD-I) and type III (FCD-III) are in this group according to Barkovich et al[3] new MCD classification.
FCD-I is a malformation presenting with abnormal cortical layering, either settle with radial cortical lamination and maturation of neurons (FCD-Ia) or the six-layered tangential cortical lamination of the neocortex (FCD-Ib). The combination of these two subgroups is categorized as FCD-Ic[16].
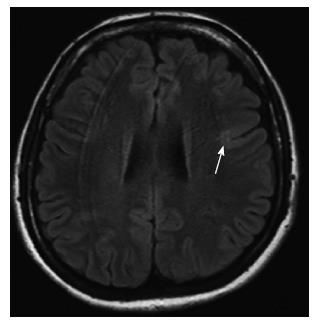
MRI examination of FCD-I cases is usually normal[15]. Hippocampal atrophy is frequently coexistent with FCD-I[17]. In some cases, prominent segmental or lobar hypoplastic/atrophic changes and decrease the volume of subcortical white matter (Figure 12), sulcal and gyral pattern abnormalities may be seen. The most common location of FCD-I is the temporal lobe[1,17].
FCD-III is divided into four subgroups; FCD-IIIa is in combination with hippocampal sclerosis. FCD-IIIb is associated with adjacent glial or glioneuronal tumors (i.e., DNET, ganglioglioma). FCD-IIIc is characterized with cortical lamination abnormalities adjacent to vascular malformations, whereas FCD-IIId is associated with any other acquired lesions in early life period (i.e., post-traumatic lesions, ischemic injury, encephalitis)[3,16,17].
MCD are an inhomogeneous group of central nervous system abnormalities and their diagnosis during the routine clinical and radiologic practice ay be challenging. The suspicion of this kind of lesions arises when a sign is observed in clinical history, radiologic imaging or EEG findings. In some cases routine MRI scanning permit detection and definitive diagnosis of these pathologies. However, in most of the cases, careful evaluation of the MR imaging that contains some special sequences and special orientations are needed for definitive diagnosis and to determine the distribution and extent of MCD.
P- Reviewer: Giovannetti G, Storto G S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Abdel Razek AA, Kandell AY, Elsorogy LG, Elmongy A, Basett AA. Disorders of cortical formation: MR imaging features. AJNR Am J Neuroradiol. 2009;30:4-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Aronica E, Becker AJ, Spreafico R. Malformations of cortical development. Brain Pathol. 2012;22:380-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, Dobyns WB. A developmental and genetic classification for malformations of cortical development: update 2012. Brain. 2012;135:1348-1369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 689] [Cited by in F6Publishing: 623] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 4. | Andrade CS, Leite Cda C. Malformations of cortical development: current concepts and advanced neuroimaging review. Arq Neuropsiquiatr. 2011;69:130-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Battal B, Sari S, Hamcan S, Akgun V. Susceptibility-weighted imaging in the diagnosis of isolated cortical vein thrombosis. Eur Neurol. 2014;71:57-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Simister RJ, McLean MA, Barker GJ, Duncan JS. Proton magnetic resonance spectroscopy of malformations of cortical development causing epilepsy. Epilepsy Res. 2007;74:107-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Woermann FG, McLean MA, Bartlett PA, Barker GJ, Duncan JS. Quantitative short echo time proton magnetic resonance spectroscopic imaging study of malformations of cortical development causing epilepsy. Brain. 2001;124:427-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Mueller SG, Laxer KD, Barakos JA, Cashdollar N, Flenniken DL, Vermathen P, Matson GB, Weiner MW. Metabolic characteristics of cortical malformations causing epilepsy. J Neurol. 2005;252:1082-1092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Andrade CS, Otaduy MC, Valente KD, Park EJ, Kanas AF, Silva Filho MR, Tsunemi MH, Leite CC. Widespread pH abnormalities in patients with malformations of cortical development and epilepsy: a phosphorus-31 brain MR spectroscopy study. Brain Dev. 2014;36:899-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Janszky J, Ebner A, Kruse B, Mertens M, Jokeit H, Seitz RJ, Witte OW, Tuxhorn I, Woermann FG. Functional organization of the brain with malformations of cortical development. Ann Neurol. 2003;53:759-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 117] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 11. | Anderson AW, Zhong J, Petroff OA, Szafer A, Ransom BR, Prichard JW, Gore JC. Effects of osmotically driven cell volume changes on diffusion-weighted imaging of the rat optic nerve. Magn Reson Med. 1996;35:162-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 82] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Rugg-Gunn FJ, Eriksson SH, Symms MR, Barker GJ, Duncan JS. Diffusion tensor imaging of cryptogenic and acquired partial epilepsies. Brain. 2001;124:627-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 213] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Eriksson SH, Rugg-Gunn FJ, Symms MR, Barker GJ, Duncan JS. Diffusion tensor imaging in patients with epilepsy and malformations of cortical development. Brain. 2001;124:617-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 218] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 14. | Flores-Sarnat L. Hemimegalencephaly: part 1. Genetic, clinical, and imaging aspects. J Child Neurol. 2002;17:373-384; discussion 384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Vurucu S, Battal B, Kocaoglu M, Akin R. Klippel-Trenaunay syndrome with hemimegalencephaly, retroperitoneal lymphangioma and double inferior vena cava. Br J Radiol. 2009;82:e102-e104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Blümcke I, Thom M, Aronica E, Armstrong DD, Vinters HV, Palmini A, Jacques TS, Avanzini G, Barkovich AJ, Battaglia G. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52:158-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1190] [Cited by in F6Publishing: 1144] [Article Influence: 81.7] [Reference Citation Analysis (0)] |
| 17. | Kabat J, Król P. Focal cortical dysplasia - review. Pol J Radiol. 2012;77:35-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Colombo N, Salamon N, Raybaud C, Ozkara C, Barkovich AJ. Imaging of malformations of cortical development. Epileptic Disord. 2009;11:194-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Pang T, Atefy R, Sheen V. Malformations of cortical development. Neurologist. 2008;14:181-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | Barkovich AJ, Kuzniecky RI, Jackson GD, Guerrini R, Dobyns WB. A developmental and genetic classification for malformations of cortical development. Neurology. 2005;65:1873-1887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 523] [Cited by in F6Publishing: 451] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 21. | Barkovich AJ. Current concepts of polymicrogyria. Neuroradiology. 2010;52:479-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Barkovich AJ, Hevner R, Guerrini R. Syndromes of bilateral symmetrical polymicrogyria. AJNR Am J Neuroradiol. 1999;20:1814-1821. [PubMed] [Cited in This Article: ] |
| 23. | Spalice A, Parisi P, Nicita F, Pizzardi G, Del Balzo F, Iannetti P. Neuronal migration disorders: clinical, neuroradiologic and genetics aspects. Acta Paediatr. 2009;98:421-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Granata T, Freri E, Caccia C, Setola V, Taroni F, Battaglia G. Schizencephaly: clinical spectrum, epilepsy, and pathogenesis. J Child Neurol. 2005;20:313-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Hayashi N, Tsutsumi Y, Barkovich AJ. Morphological features and associated anomalies of schizencephaly in the clinical population: detailed analysis of MR images. Neuroradiology. 2002;44:418-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |