Article Text
Abstract
Introduction We present the use of the Pipeline embolization device (PED) to achieve reconstruction of the right anterior circulation in a patient with a dolichoectatic internal carotid artery (ICA) and middle cerebral artery (MCA) and an associated symptomatic, large, carotid-ophthalmic segment aneurysm.
Clinical presentation A 36-year-old man presented with progressive right eye vision loss followed by sudden severe headache. Subsequent neuroimaging revealed a large right carotid-ophthalmic segment aneurysm and diffuse ectasia of the supraclinoid ICA and proximal MCA. A coil embolization of the aneurysm was performed without stent support. Over the next year, the patient experienced increasing headache and progressive bitemporal vision loss. Serial MRI showed progressive coil compaction and recanalization of the aneurysm.
Treatment The right anterior circulation was reconstructed with a total of six PEDs that extended from the distal M1 segment of the MCA proximally into the distal cavernous segment of the ICA. Follow-up angiography at 1 and 4 months demonstrated progressive complete occlusion of the aneurysm and a reorganization of blood flow to the anterior cerebral and anterior choroidal arteries. MRI and radiographic imaging provided evidence of progressive contraction of the intra-aneurysmal thrombus. The patient's headaches resolved and serial visual field examinations have demonstrated gradual improvement after treatment.
Conclusion Extensive cerebrovascular reconstructions that are not possible using commercially available endovascular devices can be achieved with Pipeline. The safety, efficacy and long term implications of such reconstructions are currently being defined.
- Pipeline embolization device
- aneurysm
- flow diversion
- parent artery reconstruction
- artery
- device
- stent
Statistics from Altmetric.com
The Pipeline embolization device (PED) is a flexible, microcatheter delivered, cylindrical, endovascular construct that is composed of a mesh of 48 individual braided strands of platinum and cobalt chromium. When unsheathed from a delivery catheter, the device expands to conform to the configuration of the parent artery. Multiple devices can be telescoped within each other either to enhance the metal surface area coverage or length of the composite construct being created. Using this “telescoping” technique, it is possible to reconstruct extensive segments of the cerebrovasculature. The metal surface area of these constructs is sufficient to disrupt intra-aneurysmal flow to the extent that aneurysms covered with the device may progress to thrombosis without the placement of embolization coils. At the same time, when placed judiciously, the porosity of the device is sufficient to allow continued perfusion of regional branches.1–4
We present a case in which a long segment of the right anterior circulation was reconstructed to address a symptomatic large carotid-ophthalmic segment aneurysm arising from a dolichoectatic internal carotid artery (ICA)–middle cerebral artery (MCA) complex. Pipeline remains an investigational device at this point and its use is restricted to approved clinical trials. The treatment described was performed under a US Food and Drug Administration approved provision to allow for the one time compassionate use of the device in this patient.
Clinical presentation
The subject, a 36-year-old man, had a long history of migraine headache, and began noticing some difficulty reading at a distance. He ultimately presented with an abrupt loss of the temporal half of his right eye visual field which progressed over a period of several hours and was accompanied by moderate to severe headache. The next day he experienced an increase in the severity of the headache. These symptoms brought him to the emergency department where CT and CT angiography (CTA) demonstrated a very large, 20-mm, right sided carotid-ophthalmic segment aneurysm projecting medially with mass effect upon both optic nerves, the optic chiasm and the basal forebrain. Imaging revealed no evidence of subarachnoid hemorrhage. No lumbar puncture was performed at this time. During this admission, he underwent conventional angiography and a successful coil embolization of the aneurysm. At that time, a dolichoectatic, fusiform enlargement of the right internal carotid artery (RICA) and M1 segment of the MCA was also noted. More than 20 embolization coils were required to achieve an acceptable packing of the aneurysm. The diameter of the ectatic parent artery precluded a stent-supported technique.
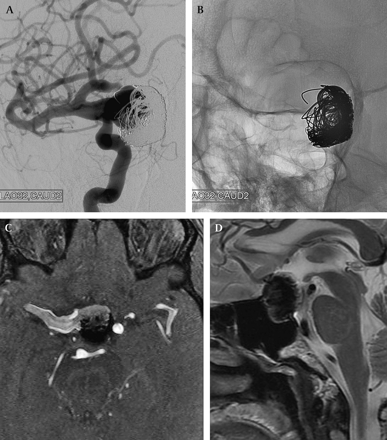
Despite the meticulous packing of the aneurysm that had been achieved, follow-up MRI at 6 and 11 months demonstrated progressive coil compaction with some growth in the overall size of the aneurysm. In addition, his headaches continued to progress as did his vision loss, which began to affect both eyes. At this point he was referred to our institution for evaluation. A cerebral angiogram was performed for treatment planning, which confirmed substantial compaction of the coil mass with aneurysm recanalization (figure 1).
Subtracted (A) and unsubtracted (B) images from a diagnostic right internal carotid artery (RICA) angiogram performed in the left transorbital oblique projection demonstrates a dolichoectatic RICA–middle cerebral artery (MCA) complex with a large (23 mm) aneurysm arising from the carotid-ophthalmic segment, projecting medially. The coil mass has compacted with marked recanalization of the medial and anterior aspect of the aneurysm. The most dilated segment of the ectatic parent supraclinoid ICA measures more than 6 mm in diameter. Axial source image (C) from an MRA shows extensive filling of the aneurysm anteriorly with compaction of the coil mass along the posterior wall of the aneurysm. The right supraclinoid ICA is markedly dilated. A sagittal T2 weighted image shows the aneurysm and coil mass in the midline with compression of the basal forebrain and extension into the sella.
The case was reviewed in our interdisciplinary cerebrovascular conference and it was concluded that open surgical and conventional endovascular options for treatment were limited. For this reason, we obtained United States Food and Drug Administration and local Institutional Review Board (IRB) approval to proceed with his treatment with the PED. After a detailed discussion with the patient and his family, they reviewed and signed an IRB approved, patient- and procedure-specific informed consent and agreed to proceed. At the time of the procedure, his daily, constant headaches had progressed to a severity that required treatment with scheduled doses of three narcotic analgesics. His vision had deteriorated to the extent that only the upper nasal quadrant of the visual fields of both eyes were preserved.
Treatment
The patient was pretreated with a loading dose of clopidogrel (600 mg) and aspirin (325 mg) 3 days before the procedure and then maintained on daily maintenance doses of clopidogrel (75 mg) and aspirin (325 mg). After femoral access was secured and the puncture site evaluated with an angiographic run, the patient was administered IV heparin to achieve an activated clotting time (ACT) of 274 s. After diagnostic angiography was performed using a standard diagnostic catheter, the guiding catheter system was manipulated into position. The platform used for the delivery and deployment of the PEDs in the present case was a triaxial system composed of an 8F 65 cm Arrow Flex Sheath (Teleflex Medical, Reading, Pennsylvania, USA), a 6F KSAW shuttle select (Cook, Indianapolis, Indiana, USA) fitted with a Check-Flo Performer (Cook) and a 105 cm 0.070″ internal diameter (ID) Neuron guiding catheter (Penumbra Inc, Mountain View, California, USA). The Neuron guiding catheter (Penumbra Inc) was manipulated into the cavernous segment of the RICA.
PED placement
A Hi-Flo Renegade microcatheter (Boston Scientific, Fremont, California, USA) was manipulated over a 0.014″ 200-cm Synchro-2 microwire through the right anterior circulation and ultimately positioned within an M2 segment of the MCA under high magnification fluoroscopic roadmap control. Then, working from distal to proximal a construct consisting of six telescoping PEDs was built, spanning from the distal M1 segment of the MCA, proximally into the distal cavernous segment of the RICA (figure 2, table 1).
Angiographic images from the treatment during which the right middle cerebral artery (MCA) and intracranial internal carotid artery (ICA) were reconstructed with Pipeline. Pretreatment subtracted images in the working angles for treatment (A, A plane; B, B plane) are chosen to display to best advantage the distal landing zone within the MCA (A) and the proximal landing zone within the distal cavernous segment of the ICA (B). Native images following reconstruction with the Pipeline embolization device (PED) show the construct extending from the distal M1 segment of the MCA (C), proximally into the distal cavernous segment of the ICA (D). Immediate post-treatment subtracted images (E, F) show patency of the construct, preserved filling of the MCA perforators, the posterior communicating arteries, the A1 segment of the anterior cerebral artery and ophthalmic artery through the interstices of the construct.
Devices implanted
Each PED is supplied loaded upon a delivery wire and constrained within a delivery sheath. The devices are loaded into the hub of the microcatheter and advanced through the microcatheter into the desired position for deployment. Once in position, the microcatheter is retracted to unsheath the device, which is stabilized proximally with the delivery wire. As it is unsheathed, the device expands toward its preset diameter to conform to the native anatomy. Once fully deployed, the delivery wire remains in position through the center of the PED and the microcatheter can be advanced over this wire, through the center of the PED, to re-establish access distal to the construct. A second device can then be loaded into the microcatheter and delivered/deployed with a predetermined degree of overlap with the preceding construct. Using this technique, a construct can be built from distal to proximal to reconstruct the targeted segment. The degree of overlap between devices is determined by the stability of the overall construct at that point and the regional branches arising from those segments in which the devices will be superimposed. Typically, between 25% and 75% of the length of the new device is overlapped with the trailing edge of the in situ construct.
The entire procedure was performed during 40.1 min of total fluoroscopic time (combined A and B planes).
Immediate angiographic and clinical results
Angiography immediately after the construct was completed demonstrated decreased inflow into the carotid-ophthalmic segment aneurysm. The fusiform, circumferential dilation of the supraclinoid ICA and MCA persisted around the construct and remained patent. The patient emerged from the procedure at his neurological baseline. Over the next 72 h, he noted a considerable improvement in his headaches. MRAs performed at 24 and 72 h after the procedure demonstrated persistent filling of the aneurysm and patency of the construct. The patient was discharged from the hospital on postoperative day #3.
Follow-up angiographic and clinical results
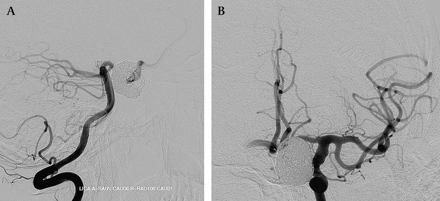
Over the next month, his headaches continued to improve and his progressive visual loss had stabilized. Angiography at 1 month demonstrated progressive thrombosis of the carotid-ophthalmic aneurysm with progressive remodeling of the supraclinoid carotid around the periphery of the construct (figure 3). Four-month follow-up angiography demonstrated the carotid-ophthalmic aneurysm to be completely occluded. The ectatic segments of the ICA and MCA had remodeled around the device (figure 4). Flow from the left ICA across the anterior communicating artery and from the posterior communicating artery provided inflow to the right anterior cerebral system. The right anterior cerebral system would only flash fill from an RICA injection. The right posterior communicating artery filled a small channel outside of the construct to provide flow to the anterior choroidal artery as well as the anterior cerebral artery (figure 5).
1-month follow-up subtracted angiogram with replication of the working angles (A, B) shows near complete thrombosis of the ophthalmic aneurysm and progressive contraction/collapse of the dolichoectatic supraclinoid internal carotid artery (ICA) as it remodels around the construct. The perforators arising from the M1 segment all remain patent as does the ophthalmic artery. The A1 segment of the anterior cerebral artery still demonstrates anterograde filling, however, it is not as robust as on the immediate post-treatment angiogram. In addition, the posterior communicating artery, demonstrated on the immediate post-treatment angiogram, no longer fills from the internal carotid injection.
4-month follow-up subtracted angiogram with an attempted replication of the working angles (A, B). Due to a change in the relationship between the Pipeline embolization device construct and coils, likely secondary to the resorption of thrombus and the resolution of regional mass effect, the same anatomical projection was no longer possible. The aneurysm has undergone complete thrombosis in the interim. The perforators arising from the M1 segment of the middle cerebral artery (MCA) and the ophthalmic artery remain patent and fill antegrade. The posterior communicating artery and A1 segment of the anterior cerebral artery (ACA) are no longer visualized with a ‘physiologic’ right internal carotid artery (RICA) injection. A very firm injection of the RICA did flash fill the ipsilateral A1 and minimally opacify the ACA system. Further angiography was performed to demonstrate the supply to the ACA system.
4-month follow-up subtracted angiograms from catheters positioned within the left vertebral (A, lateral projection) and left internal carotid artery (B, posterior–anterior (PA) projection), demonstrate patency of the entire anterior cerebral artery (ACA) system. The right posterior communicating artery fills from posterior to anterior from the vertebral injection (A). This supplies a small crescent shaped channel along the dorsal aspect of the internal carotid artery (ICA) construct to fill the anterior choroidal artery and A1 segment of the ACA. With the exception of this channel, the ectatic segment of the right internal carotid artery (RICA) has collapsed/contracted circumferentially about the PED construct. Additional supply to the ACA system is provided by robust filling across the anterior communicating artery from the left ICA injection (B). These images demonstrate the dynamic alteration of the cerebrovascular flow patterns that can occur after PED reconstruction.
During the 4-month follow-up angiogram, it was impossible to recreate the anatomical relationship between the PED construct and the coil mass, secondary to a change in the regional mass effect created by the aneurysm and associated thrombus mass. The interval thrombosis of the saccular aneurysm and subsequent thrombus resorption could be appreciated on the lateral native views from angiography as a collapse or contraction of the intra-aneurysmal coil mass (figure 6). Subsequent MRI performed immediately after the 4-month follow-up angiogram demonstrated a slight but measurable decrease in the collective aneurysm (thrombus coil) mass, most likely secondary to a gradual resolution of the intra-aneurysmal thrombus. The superior–inferior dimension of the aneurysm (thrombus coil) mass measured 22.5 mm before treatment, decreasing to 20.6 mm at the 4-month follow-up. Axial T2 weighted sequences showed a similar reduction in the axial dimensions of the aneurysm (thrombus coil) mass. Although the absolute measurements showed only small changes, these corresponded to a substantial reduction in the overall volume of the aneurysm (4780–3245 cubic mm).
Native images obtained in the lateral projection before treatment (A) and at 4-month follow-up (B) show the ‘restorative’ potential of Pipeline embolization device treatment. After the aneurysm has completely thrombosed these images suggest that the thrombus has begun to resorb and the coil mass has started to circumferentially collapse/contract. The dashed line traces the arc of the C-shaped configuration of the compacted coil mass which has become more acute in shape during the intervening months after treatment. The alignment of the osseous landmarks (eg, the margins of the sphenoid sinus) confirms an exact replication of the imaging projection during the follow-up angiogram.
Clinically, his headaches have nearly completely resolved and he is only on a single analgesic medication, which he uses sparingly. His vision has also started to gradually improve with restoration of the nasal fields of the left eye and an expansion of the upper nasal quadrant of the right eye. Although no visual field exam was available from the weeks immediately prior to his treatment, the patient subjectively states that his visual fields have improved from his immediate preprocedural baseline.
Conclusions
The present case report illustrates several important concepts related to the application and potential utility of the PED.
Pipeline provides a potentially much more efficient means by which to achieve a complete and durable occlusion of intracranial aneurysms.
Pipeline enables reconstructions of the cerebrovasculature tha are far more extensive than what had been possible with the predicate endovascular devices.
It is possible to build Pipeline constructs judiciously across eloquent branch vessels and perforators without jeopardizing their patency. While some vessels maintain direct, antegrade patency and fill through the interstices of the construct, others, which have competitive flow, may reorganize their directionality with time and as the construct matures.
The efficiency of Pipeline for the treatment of large and giant aneurysms: an evaluation of the Potential advantages
The efficiency of aneurysm reconstruction with the PED compares favorably with conventional coil embolization and the advantages of this efficiency are manifest in several ways. First, with respect to the actual treatment session itself, the overall procedural times and fluoroscopic times are likely to be considerably lower than with predicate techniques. The present PED reconstruction was achieved with only 40 min of total fluoroscopic time (for both the A and B planes combined) for the entire case. Considering that during the initial coil embolization procedure, more than 20 coils were placed, it is entirely conceivable that if even 2 min (per imaging plane) were required for the placement and detachment of each coil, that the coil placement alone could require double the fluoroscopic time of the entire PED reconstruction procedure. The radiation exposure required for serial angiographic follow-up and very possibly the subsequent serial re-coiling continues to add to the overall accumulated dose.5 Second, the overall cost of aneurysm treatment may be substantially reduced with the PED. Given that the cost of a single embolization coil in the USA ranges between US$800 and 3000, it is conceivable that the implantable costs alone for an aneurysm of this size could be between US$16 000 and 40 000 for the initial procedure, with additional costs incurred each time a recurrence requires another session of coil embolization. Depending on the cost per unit, it is possible that even with the use of multiple devices (as in the present case) PED treatment could represent a much more economical approach to these lesions. This is particularly evident if one considers the potential cost of multiple retreatments in terms of serial imaging surveillance, hospital readmission, additional implantable costs, anesthesia and time in the catheterization lab. Finally, while the safety of Pipeline reconstruction has not yet been quantified, it is possible that a single curative procedure would have a greater margin of peri-procedural safety than multiple treatments/retreatments and continued invasive imaging surveillance.
Vascular reconstruction with Pipeline: what can be done?
The vascular reconstructions that are technically achievable with Pipeline exceed what has been possible using the predicate commercially available intracranial self-expanding stents (eg, Neuroform, Boston Scientific; Enterprise, Cordis Neurovascular, Warren, New Jersey, USA). Using the described technique of telescoping multiple devices to bridge the diseased anatomy, it is technically reconstruct extensive segments (>5 cm) of the cerebrovasculature. Using the PED, it is technically feasible to reconstruct the contiguous vascular segments that constitute the ‘anterior’ or ‘posterior’ circulations. Thus, as this technology becomes widely available, new treatments will be possible. Aneurysms and diseased vascular segments that were previously unapproachable will be able to be addressed (at least from a purely technical standpoint) with Pipeline. Correspondingly, the emerging experience with these devices will define a new frontier of endovascular aneurysm therapy.
Crossing major branch vessels and perforators with the PED
When applied judiciously, the existing data suggest that it is possible to build PED constructs across major branch arteries and perforators without clinical consequence. The limits of these reconstructions are still being defined. From a physiological standpoint, there are two general categories of branch vessels, those that lack significant collateral support and those that have collateral support. For those segments of the parent artery that give rise to vessels lacking collateral support, we have attempted to limit coverage to a single device when possible. In some cases, it is even feasible to oversize the device such that the elongation of the braided mesh pattern will lead to a more porous cell structure and less overall metal surface area coverage in these regions.
When vessels have adequate collateral support (eg, the A1 segment of the anterior cerebral artery (ACA) and ophthalmic artery in the present case) they may be covered with multiple PEDs with less concern. In these cases, the flow may remain antegrade through the interstices of the construct, or as the construct matures, a reorganization may take place such that the competitive inflow gradually takes over and flow in the branch becomes retrograde. In the present case, the A1 segment of the ACA filled from the RICA injection immediately after the construct was placed and at 1-month follow-up angiography. However, with time, as the carotid-ophthalmic aneurysm completely thrombosed and the construct began to become incorporated into the parent artery, competitive flow from the left anterior cerebral system (and from the posterior communicating artery) became the dominant sources for flow to the right ACA system (figure 5).
Reorganization of flow patterns after Pipeline
The reorganization of the cerebral blood flow that occurs after the PED construct is placed is likely governed by physiological pressure gradients. These pressure gradients (or ‘outflow demands’) function to set up physiological currents of flow, which may mature into established channels (‘flow reconstructed neovessels’), which may unite regional vessels that arise outside of the construct. In the present case example, the posterior communicating artery (PCOMM), anterior choroidal artery and right A1 segment were anatomically excluded from the ICA by the PED construct. Immediately after the construct was in place, flow in the PCOMM was anterior to posterior in direction and the posterior circulation was easily opacified with a RICA injection. However, by 1 month, the PCOMM was no longer visualized during RICA angiography. At 4 months, this arrangement persisted. Injection of the left vertebral artery at 4 months resulted in robust posterior to anterior filling of the PCOMM which supplied a small channel around the outside of the construct. This crescentic, ‘flow reconstructed’ channel along the outside of the ICA segment of the construct provided filling of the anterior choroidal artery proximally and the A1 segment of the anterior cerebral artery more distally. This thin channel outside of the PED construct represents the only remnant of the dolichoectatic ICA–MCA complex that had subsequently collapsed/contracted circumferentially to remodel about the periphery of the construct.
While this reorganization of the regional vascular anatomy allows continued perfusion of eloquent branch vessels arising from the ICA, it must be acknowledged that the implications of this type of anatomical configuration are incompletely understood at this point. On some level, this process is anatomically similar to a ‘type II endoleak’ after aortic stent graft placement. However, even with the substantial existing experience with aortic stent grafts, the significance of these ‘type II endoleaks’ remains incompletely understood and controversial.6–8 Although we hypothesize that the associated shear stress and overall flow transit through this vascular segment would be markedly reduced after the Pipeline reconstruction, we acknowledge that continued monitoring with MR imaging is indicated.
Anatomical restoration: moving beyond angiographic occlusion
In addition to creating a complete angiographic exclusion of the lesion, Pipeline reconstruction provides potential for ‘anatomical restoration.’ After the aneurysm is excluded from the circulation, the thrombus mass within the lesion may resorb, leading to a decrease in the mass effect associated with the lesion. In the present case, the degree of ‘anatomical restoration’ that could be achieved was limited by the large pre-existing intra-aneurysmal coil mass. Despite the preservation of mass effect created by the coil mass, at the time of 4-month imaging follow-up it was evident that there had been some resolution of the mass effect created by the aneurysm. During cerebral angiography, it was impossible to replicate the orientation of the PED construct, the coil mass and the osseous landmarks due to the interval change in the anatomical relationship of these objects, likely secondary to the resolution of regional mass effect (figure 6).
This process of ‘restoration’ represents an important component of endovascular aneurysm therapy, particularly for large and giant aneurysms which frequently present with symptoms of mass effect. The benefits of this restorative process in the present case may have been manifest as the resolution of headache and improvement in the pre-existing visual deficits.
Limitations
This report represents a single case, and although the results were favorable, they cannot be widely applied to complex aneurysms in other locations (or in other patients for that matter). We report our results to demonstrate that PED reconstruction represents a potential option that may be considered by physicians who encounter patients with similar complex lesions that lack other good treatment options. It is important that if the PED is being considered as a potential option for curative therapy, that operators avoid pursuing ‘partial treatment’ using adjunctive endovascular stents (eg, Neuroform (Boston Scientific) and Enterprise (Cordis Neurovascular)), since these devices can impair the future delivery and deployment of PED. Finally, our experience with the PED is still very early, and the long term implications of these extensive endovascular reconstructions remain largely unknown. It is important to recognize that the PED (at this point) remains an investigational device within the USA and as such it is only approved for use within the context of ongoing clinical trials.
Conclusions
Extensive endovascular reconstructions are possible with Pipeline. These reconstructions may allow curative treatment of aneurysms that cannot be adequately treated with the present spectrum of endovascular devices. Although the available results have been encouraging, the safety, efficacy and long term implications of such reconstructions are still being defined.
Footnotes
Competing interests PN is a stockholder and consultant to Chestnut Medical.
Patient consent Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.