Article Text
Abstract
Background Flow diverters are increasingly used for the treatment of intracranial aneurysms. Understanding cavernous internal carotid artery (cICA) tortuosity may help to predict procedural complexities of deploying flow diverters.
Methods Pipeline embolization device (PED) neurointerventions for ICA aneurysms proximal to the ICA termination were reviewed. Cavernous ICA tortuosity was measured as a ratio D/AP, where D=height difference of the anterior and posterior genus, AP=sum of the angles of the anterior (A) and posterior (P) genus. Four types of cICA tortuosity were proposed. An analysis of variance regression and Fisher's exact test were performed to analyze differences among the types.
Results Cavernous ICA tortuosity was categorized into minimal (type I, n=28), moderate (type II–III, n=29), and severe (type IV, n=26). The groups were comparable for patient age (mean ± SEM years, type I: 55.6±10.4, II–III: 56.4±14.4, IV: 55±12.8) and aneurysm size (mean±SEM mm, type I: 6.25±3.5, II–III: 7.6±4.9, IV: 9.11±4.9). Analysis of variance demonstrated significant differences in procedural fluoroscopy time (mean ± SEM min, type I: 29.8±8.4, II–III: 44.9±34.1, IV: 52.6±17.2, p<0.005) and mean ± SEM D/AP (type I: 0.008±0.0008, II–III: 0.141±0.07, IV: 0.482±0.365, p<0.0001). Procedural complexity was also statistically significant (p<0.005) with 4%, 28%, and 35% of cases in types I, II–III, and IV, respectively, requiring intraprocedural PED removal or balloon post-processing of the implanted PED.
Conclusions We propose a classification system for cICA tortuosity based on measurements of the anterior and posterior genu geometry. This classification correlates strongly with markers of PED procedural complexity and may be helpful in pre-procedure prognostication.
- Aneurysm
- Artery
- Catheter
- Flow Diverter
- Intervention
Statistics from Altmetric.com
Introduction
Parent vessel reconstruction with flow diverting technology, such as the Pipeline embolization device (PED; Covidien Vascular Therapies, Mansfield, Massachusetts, USA), is rapidly becoming the preferred endovascular modality for cerebral aneurysms, particularly those that are large and giant, fusiform or wide-necked along the proximal internal carotid artery (ICA).1–4 The safety and cost-effectiveness of PED has led to its widespread use and application for other smaller wide-necked aneurysms throughout the intracranial circulation.5–9 The complexity of PED procedures can be highly variable, at times requiring removal or ‘corking’ of a partially opened PED during the deployment process or after processing of an implanted PED with balloon or microwire.10
Successful endovascular treatment of intracranial aneurysms relies on telescoping catheter support systems to provide safe stable access to the intracranial circulation. Modern neurointerventional procedures with larger device delivery systems such as the PED and tortuous anatomy have resulted in increasing use of more robust triaxial systems with hyperflexible distal intracranial support catheters at the cornerstone of these set-ups.11 ,12 Despite optimization of the access platform, tortuosity of the cerebrovasculature can still lead to technical failures that necessitate complex alternative approaches.13–15 We hypothesize that the degree of tortuosity of the ICA may help to predict the procedural complexities of deploying flow diverters such as the PED. In this report, we assessed the tortuosity of the cavernous ICA (cICA) seen in PED treatments by analyzing the geometry of the anterior and posterior genus. We subsequently categorized the various types of tortuosity and correlated this with the observed complexity of the PED procedures. To our knowledge, this is the first study that functionally classifies cICA tortuosity and demonstrates its validity as a marker for PED procedural complexity.
Patients and methods
Patient selection
We retrospectively reviewed a prospective, single-center aneurysm database from October 2011 to April 2013, identifying all patients with intracranial ICA aneurysms who underwent endovascular treatment using the PED with the Navien distal intracranial catheter access platform.
Endovascular procedure
Embolization procedures were performed as previously described.8 ,11 Briefly, all patients were treated preoperatively with a dual antiplatelet regimen consisting of aspirin 325 mg daily and clopidogrel 75 mg daily for 7 days before the intervention. The degree of P2Y12 receptor inhibition was not routinely tested. All procedures were performed with systemic anticoagulation using heparin with a 5000 U bolus at the start of each case followed by an intraprocedure rebolus of 1000 U at each additional hour. The goal of each procedure was complete coverage of the aneurysm neck or fusiform/diseased vessel segment with the PED and maximum vessel wall apposition along the length of the PED. A triaxial system was used through femoral access. This consisted of a 6 French Flexor Shuttle sheath (Cook Medical, Bloomington, Indiana, USA), a 5 French Navien distal intracranial catheter (Covidien Vascular Therapies, Mansfield, Massachusetts, USA) serving as the guide catheter,11 and a Marksman microcatheter (Covidien Vascular Therapies). The distal PED was opened in the ipsilateral ICA or, more commonly, in the ipsilateral M1 segment. Proper device expansion and deployment was assessed with native fluoroscopy. If a partially deployed PED did not open properly or was malpositioned, the device was corked and removed.10 Control DSA was performed immediately after deployment and at 5 and/or 10 min after deployment to confirm patency of the parent vessels and to rule out intraluminal thrombus. In cases that required post-processing of the implanted PED, a HyperForm or HyperGlide balloon (Covidien Vascular Therapies) was used.
Cavernous ICA (cICA) tortuosity classification and measurements
cICA tortuosity was classified into four types based on the geometry of the anterior and posterior genus, as shown in Figure 1. Type I has open configurations/angles of the genus with subcategory determined by the posterior genu angle where IA is greater than 90° and IB is equal to 90°. Type II is characterized by a closed configuration of the anterior genu reflected by a more acute angle of the genu compared with type I. Type III is defined by the posterior deflection of the posterior genu giving it a buckled appearance. Type IV is the most tortuous cICA with a shape characteristic of the Simmons-style angiography catheter where the posterior genu is buckled superiorly compared with the anterior genu. Mild, moderate, and severe cICA tortuosity were defined as type I (A or B), type II–III, and type IV, respectively.
Classification of cavernous internal carotid artery (cICA) tortuosity. Type I has open configurations/angles of the genus with subcategory determined by the posterior genu angle, where IA is greater than 90° and IB is equal to 90°. Type II is characterized by closed configuration of the anterior genu reflected by a more acute angle of the genu compared with type I. Type III is defined by the posterior deflection of the posterior genu giving it a buckled appearance. Type IV is the most tortuous cICA with characteristic Simmons catheter shape, where the posterior genu is buckled superiorly compared with the anterior genu.
The cICA tortuosity was represented as a ratio D/AP as shown in figure 2. D is the height difference of the anterior and posterior genus from the peak of the posterior genu and the trough of the anterior genu. For types IA and IB where the horizontal segment lacked any tortuosity, D was set at 1 mm. AP is the sum of the angles of the anterior (A) and posterior (P) genus. The anterior genu angle is measured with the vertex originating from the anterior wall and the vectors along the long axis of the ICA. The posterior genu angle is measured with the vertex originating from the posterior wall and the vectors along the long axis of the ICA.
Measurement of cavernous internal carotid artery (cICA) tortuosity. The cICA tortuosity was represented as a ratio D/AP. D is the height difference of the anterior and posterior genus, measured from the peak of the posterior genu to the trough of the anterior genu. For types IA and IB where the horizontal segment lacked any tortuosity, D was set at 1 mm. AP is the sum of the angles of the anterior (A) and posterior (P) genus. The vertices of the angles originate from the anterior wall for the anterior genu and the posterior wall for the posterior genu. The vectors are drawn along the long axis of the ICA.
Data collection
Data on patient demographics and anatomic characteristics of the aneurysm were collected. Procedural data analyzed include aortic arch type, cervical ICA tortuosity (defined as a 90° turn, hairpin turn, or corkscrew loop), cICA tortuosity, fluoroscopy time, and level of complexity (including PED corking and balloon post-processing). Data were presented as counts or mean±SEM.
Statistical analysis
Spearman's rank correlation coefficient was used to assess the length of procedural fluoroscopy time as it relates to aneurysm size and cICA tortuosity (D/AP). Differences among the cICA types were analyzed using a one-way analysis of variance regression test for comparison of means and Fisher's exact test for comparison of counts. The following variables were analyzed: procedural fluoroscopy duration, sum of the angles of the anterior and posterior genu, distance between the height of the posterior genu and the trough of the anterior genu, degree of cICA tortuosity (D/AP), aneurysm size, and procedural complexity. A probability value of <0.05 was considered statistically significant.
Results
Patient demographics and aneurysm characteristics
Between October 2011 and April 2013, 83 cases of intracranial ICA aneurysms were treated with PED using the Navien distal intracranial catheter access platform. The patient demographics and aneurysm characteristics are presented in table 1. Cases were equally distributed among the three groups of tortuosity: minimal (type I, n=28), moderate (type II–III, n=29), and severe or ‘Simmons-type’ (type IV, n=26). The three groups were comparable for patient age (mean ± SEM years, type I: 55.6±10.4, II–III: 56.4±14.4, IV: 55±12.8), aneurysm size (mean±SEM mm, type I: 6.25±3.5 mm, type II–III: 7.6±4.9 mm, type IV: 9.11±4.9 mm), and gender (female, type I: 89%, II–III: 93%, IV: 100%). Most of the aneurysms in all three groups were cavernous or paraophthalmic/clinoidal.
Patient demographic data and aneurysm characteristics
Procedural characteristics
There were no significant differences in aortic arch type (p=0.635) and cervical ICA tortuosity (p=0.578) between the three groups (table 2), thus any statistical differences in procedural fluoroscopy time (FT) and technical complexity were unlikely to be related to the proximal vascular anatomy. Analysis of variance showed significant differences in genu geometry between the three groups. The mean sum of the anterior and posterior genu angles (AP) was obtuse in type I (122.6±13.2°) and acute in type IV (31.97±15.3°) (p<0.0001). The anterior–posterior genu distance (D) was 1 mm in type I, 7.1±0.9 mm in type II–III, and 10.8±1.7 mm in type IV (p<0.0001). Overall, the degree of cICA tortuosity (D/AP) was also statistically different (type I: 0.008±0.008 mm; type II–III: 0.141±0.07 mm; type IV: 0.482±0.365 mm, p<0.0001).
Procedural characteristics
Using the Spearman rank correlation test, the procedural FT was found to be significantly dependent on the cICA tortuosity (r=0.567, p<0.0001) and aneurysm size (0.34, p<0.005). Comparison of the cICA types using analysis of variance regression showed a significantly longer mean FT in type IV (52.6±17.2 min) than in type II–III (44.9±34.1 min) and type I (29.8±8.4 min), p<0.005. Figure 3 shows an example of the effect of the cICA tortuosity on procedural FT with a patient who had bilateral paraophthalmic aneurysms treated with PED, where the aneurysm with type IV cICA required four times as long to deploy the PED as the other aneurysm with type I cICA. Additionally, the difference in the procedural complexity between the types was statistically significant (p<0.005) with 4%, 28%, and 35% of cases in types I, II–III, and IV, respectively, requiring intraprocedural PED removal or balloon post-processing of the implanted PED. Of the 83 cases in this series, four were unsuccessful PED implantations—three in type II–III cICAs and one in type IV. Figure 4 illustrates the case of type IV cICA and failed PED deployment. The image shows stretching of the proximal end of the PED as a result of the paradoxical movements associated with Simmons-type anatomy.
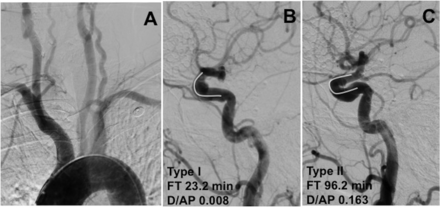
Effect of cavernous internal carotid artery (cICA) tortuosity on procedural fluoroscopy time in deployment of the Pipeline embolization device. (A) Arch aortogram, anterior–posterior view, demonstrating type I arch. (B) Left common carotid DSA, lateral view, demonstrating 8 mm paraophthalmic aneurysm and type I cICA. The white curve demonstrates the open configuration of the anterior genu, characteristic of type I cICA without tortuosity. (C) Right common carotid DSA, lateral view, demonstrating 5 mm paraophthalmic aneurysm and type IV cICA. The white curve demonstrates the flattened anterior genu with an acute angle representing moderate (type II) cICA tortuosity.
Case of unsuccessful Pipeline embolization device (PED) deployment in type IV cavernous internal carotid artery (cICA). (A) Left common carotid DSA, lateral view, demonstrating type IV cICA with the characteristic Simmons shape and two aneurysms (paraophthalmic and cavernous). (B, C, D) Intraprocedural native single-shot fluoroscopy views of PED deployment. White arrows highlight the constrained proximal end of the device that could not be opened as a result of the paradoxical microcatheter movements similar to the Simmons class of angiographic catheters.
Discussion
Based on the Bouthillier classification of the ICA segments, the cavernous segment of the ICA begins at the petrolingual ligament and extends to the dural ring.16 It has a characteristic S shape defined by two distinct bends, an anterior genu and posterior genu, connected by a horizontal segment.17 This S shape of the ICA has also been referred to as the carotid siphon throughout the medical literature. Since Moniz coined the term in 1927, the anatomic boundaries of the siphon have been ambiguous.18 Krayenbuehl and Yasargil subsequently reported their observations of the markedly varied shapes of the carotid siphon: a U shape, a V shape, Arc shape, Omega shape, a double siphon, a megasiphon, or a dolichosiphon.19 A few studies have analyzed the carotid siphon geometry as it relates to cerebral aneurysms. In a comparison of 59 patients with posterior communicating artery aneurysms with 63 control patients without aneurysms, Silva Neto and colleagues concluded that a narrower carotid siphon was associated with aneurysm formation.20 Computational model analysis of 65 aneurysms performed by Sangalli and colleagues showed that aneurysms proximal to the ICA termination had narrower carotid siphons than aneurysms at the ICA termination or beyond.21
Although studies have demonstrated the relationship between cICA anatomy and the formation of specific aneurysms, few have examined how these variations affect the endovascular case complexity. Several markers of cICA or carotid siphon tortuosity have been identified. These include a closed configuration of the anterior genu, an acute angle of the siphon, exaggerated loops of the genus, or a sharply sloping horizontal segment.17 ,22 ,23 Jiang and colleagues provided the first report of an effect of these anatomic variations on procedural outcomes with their initial experience with middle cerebral artery stenting for symptomatic M1 stenosis.24 In this series, the authors graded carotid siphon tortuosity into three types based on the U and V shapes of the Krayenbuehl classification and considered the technical success rate of stenting (100% (17/17), 100% (18/18), and 85.7% (6/7) for types I, II, and III, respectively) to be a function of the siphon tortuosity.24 ,25 More recently, in a retrospective review of their experience with the jailing technique using the Neuroform EZ stent (Stryker Neurovascular, Kalamazoo, Michigan, USA), Yoon et al found that the acuteness of the carotid siphon significantly influenced the interference between the coiling microcatheter and the stenting microcatheter.23 In the presence of microcatheter interference the mean carotid siphon angle was 51.2° compared with 88.3° in cases without interference.
Our report is the first dedicated analysis of cICA tortuosity and its effect on the procedural complexity in neurointerventions with PED deployment. We designed a quantitative measurement of cICA tortuosity as the ratio D/AP, where D is the height difference of the anterior and posterior genus and AP is the sum of the angles of the anterior and posterior genus. Although the angle measurements may be subject to interuser variability, we still found a statistically significant dependence of the PED procedure fluoroscopy time on the degree of cICA tortuosity (r=0.567, p<0.0001), as well as the aneurysm size (r=0.34, p<0.005), using the Spearman rank correlation test.
Considering these findings, we proposed a four-tier classification system for the cICA tortuosity based on the geometry of the anterior and posterior genus (figure 1A). The degree of tortuosity was then categorized into minimal (type I, n=28), moderate (type II–III, n=29), and severe or ‘Simmons-type’ (type IV, n=26). The three groups were comparable for patient age (mean±SEM years, type I: 55.6±10.4, II–III: 56.4±14.4, IV: 55±12.8), aneurysm size (mean mm, type I: 6.25±3.5 mm, type II–III: 7.6±4.9 mm, type IV: 9.11±4.9 mm), aortic arch type (p=0.635), and presence of cervical ICA tortuosity (p=0.578). The groups were significantly different in their degree of tortuosity (D/AP) with a type IV having the highest mean value of 0.482±0.365 compared with 0.141±0.07 and 0.008±0.0008 for type II–III and type I, respectively (p<0.0001). These results validated the proposed classification system.
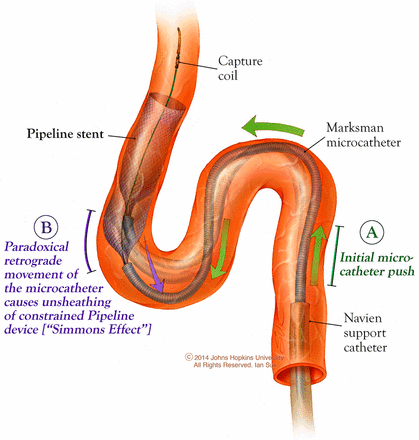
Markers of PED procedural complexity were then analyzed as they related to the cICA tortuosity classification. The mean fluoroscopy time was longer in type IV (52.6±17.2 min) than in type II–III (44.9±34.1 min) and type I (29.8±8.4 min), p<0.005. Figure 3 illustrates an example of the effect of the cICA tortuosity on the PED procedural fluoroscopy time. A patient with type I aortic arch had an 8 mm paraophthalmic aneurysm with a type I cICA and a 5 mm paraophthalmic aneurysm with a type II cICA. The PED deployment for the 5 mm aneurysm took about four times as long as the deployment for the 8 mm aneurysm and required balloon post-processing of the proximal end of the device along the anterior genu. Additional indicators of PED procedural complexity, such as occurrences of intraprocedural PED removal, balloon post-processing of the implanted PED, and case failure, occurred at a significantly higher rate in type IV with 35% of cases versus 28% in type II–III and 4% in type I, p<0.005. Figure 4 presents the one case of type IV cICA with unsuccessful PED deployment. We have found that in type IV cICAs the exaggerated superior buckling of the posterior genu relative to the anterior genu can magnify the complexity of a PED deployment by causing a paradoxical behavior of the microcatheter movements required for PED opening and apposition. In the Simmons class of angiographic catheters, pushing the catheter results in retraction rather than advancement of the distal tip. Similarly, in type IV cICAs, as the PED is deployed across the anterior genu, pushing the Marksman microcatheter forward paradoxically stretches the PED instead of compressing it. Figure 5 is a pictorial demonstration of this ‘Simmons’ effect. Subsequently, these back and forth ‘wagging’ movements of the microcatheter are not reliably transferred in these scenarios. Thus, we refer to the shape of the type IV cICA as a ‘Simmons’ type. Additionally, this can cause distal device migration/prolapse if this effect is not accounted for at the beginning of deployment. Further distal positioning of the Navien guide catheter over the posterior genu can ameliorate these paradoxical effects seen in the type IV Simmons-like cICAs. With a more distal position past the posterior genu, a more direct one-to-one translation of the forces from the microcatheter to the device can be expected.
‘Simmons’ effect in a type IV cavernous internal carotid artery (cICA). In the type IV cICA, the exaggerated superior buckling of the posterior genu relative to the anterior genu causes a paradoxical behavior of the microcatheter movements required for Pipeline embolization device (PED) opening and apposition. In the Simmons class of angiographic catheters, pushing the catheter results in retraction rather than advancement of the distal tip. Similarly, in the type IV cICA, as the PED is deployed across the anterior genu, pushing the Marksman microcatheter forward paradoxically stretches the PED instead of compressing it. This paradoxical movement is the ‘Simmons’ effect. Additionally, this effect can cause distal device migration/prolapse if it is not accounted for at the beginning of deployment.
In this report, we present a grading system of cICA tortuosity that is simple to understand, easy to apply, and with statistical significance for procedural complexity and outcomes. This system was designed for use by all neurointerventionalists with access to lateral planar DSA images. Although 3D morphometry probably provides additional qualitative and quantitative understanding of the cICA anatomy, it also adds a significant layer of complexity to a grading system and requires adjunct imaging software. Added complexity, without significant practical benefit, makes a grading system cumbersome and limits its clinical utility. In our experience, the additional cICA tortuosity seen with 3D imaging, such as the medial–lateral course of the horizontal cavernous segment, does not add measurable procedural complexity. This type of tortuosity is commonly associated with the morphology seen in a type III or IV genus and rarely found in a type I or II genus. Thus, the planar images alone provide sufficient information for a rapid and meaningful understanding of the cICA tortuosity. The grading system proposed in this report augments this understanding with a simple quantifiable analysis of the different cICA types.
Conclusion
As modern neurointerventions continue to evolve with innovative devices requiring larger delivery systems, understanding the tortuosity of the cICA may help to predict procedural complexities and lead to technical success. We demonstrated a classification system for cICA tortuosity based on measurements of the anterior and posterior genu geometry. This classification correlates strongly with markers of PED procedural complexity and may be helpful in planning access and prognostication of procedure success. This classification of cICA tortuosity may be applied to other types of anterior circulation neurointervention.
Acknowledgments
We thank Ian Suk, BSc, BMC from the Department of Neurosurgery and Art as Applied to Medicine, Johns Hopkins University School of Medicine, for assistance with preparation of the illustrations.
References
Footnotes
Contributors L-ML drafted the manuscript and critically revised the manuscript for important intellectual content. GPC assisted in critically revising the manuscript. BJ helped to draft the manuscript. CU assisted with the data collection and analysis. JH and RJT critically reviewed the important intellectual content. ALC conceived the idea for the manuscript and critically reviewed the important intellectual content. All authors read and approved the final manuscript.
Competing interests ALC is a proctor for the Pipeline embolization device (Covidien, Mansfield, Massachusetts, USA), Surpass flow diverter (Stryker, Kalamazoo, Michigan, USA), and flow redirection endoluminal device (MicroVention, Tustin, California, USA). He is also a consultant for Covidien and Stryker. GPC is a consultant for Covidien. The other authors have no conflict of interest. No author received financial support in conjunction with the generation of this submission.
Ethics approval This study was conducted with the approval of the Johns Hopkins medicine institutional review board.
Provenance and peer review Not commissioned; externally peer reviewed.