Article Text
Abstract
Background and purpose Adjunctive devices are commonly employed in the treatment of wide necked cerebral aneurysms. Balloon remodeling and permanent stent implantation may lead to thromboembolic complications or the need for antiplatelet use. A temporary stent that does not lead to complete flow arrest may be beneficial.
Methods We studied 20 New Zealand white rabbits in whom aneurysms were created using elastase and ligation of the right common carotid artery. The aneurysms were then embolized with bare platinum coils along with adjunctive treatment using the Comaneci device or the Hyperglide balloon. Assessments were made for endothelial injury using scanning electron microscopy (SEM) and light microscopy.
Results 20 rabbits of mean±SD weight 3.1±0.2 kg were studied. Twelve rabbits were treated with the Comaneci device and eight with the Hyperglide balloon. There were no substantial differences on SEM or light microscopy in the subacute and chronic phase to suggest the Comaneci device caused endothelial injury.
Conclusions The Comaneci device is a new adjuvant treatment for bridging of wide necked aneurysms with the advantage of averting flow arrest during deployment. There does not appear to be any evidence of significant endothelial damage during deployment in preclinical studies.
- Aneurysm
- Stent
- Device
Statistics from Altmetric.com
Introduction
The utilization of endovascular therapies with adjunctive devices to treat ruptured and non-ruptured aneurysms is being employed more frequently with improvement in device technology.1 Patients harboring wide necked aneurysms traditionally pose a higher risk of treatment with endovascular therapy due to the requirement for an adjuvant device. This can be particularly challenging in patients with ruptured cerebral aneurysms in whom microsurgical clipping may be a technical challenge either due to the location of the aneurysm or neurological status of the patient.2 Although stents have been implanted in ruptured aneurysms, there appears to be a high incidence of hemorrhagic complications due to the need for dual antiplatelet agents.3 Balloon remodeling is more commonly used but leads to flow arrest to the territory of the brain that may place the patient at a higher risk for ischemic injury. The Comaneci device represents a unique flexible mesh that allows for temporary neck bridging during embolization of the aneurysm while averting flow arrest.
Methods
Device description
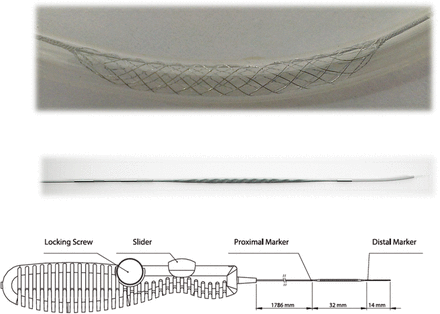
The Comaneci device was designed to bridge the neck of an aneurysm during coil embolization. It consists of a 183 cm length core wire with a diameter of 0.5 mm. The stent device is 32 mm in length but when fully expanded foreshortens to 20 mm with a 3 mm diameter (figure 1). The distal end has a 12 mm soft flexible tip to assist the operator in safe placement of the device with better fluoroscopic visualization. The device is delivered through a microcatheter with a 0.021 inch internal diameter.
The top panel shows the Comaneci device expanded while the middle panel exhibits the device constrained. The bottom image is a depiction of the slider that is used to open the device where two marker bands are visible on fluoroscopy to view the device in its entirety.
Animal model and procedure techniques
For the purpose of this experiment the New Zealand white rabbit was employed using the elastase model. The goal of the study was to assess the safety and response of the Comaneci device compared with the Hyperglide balloon as a neck bridging device for aneurysms. The objectives of the study were to assess endothelial injury of the parent vessel as well as the absence of intraluminal filling of the aneurysm. This was done with light microscopy (LM) to evaluate the degree of healing of the aneurysm and scanning electron microscopy (SEM) for an ‘en face’ assessment of the luminal surface.
Aneurysm surgical procedure
The aneurysms were created by surgical ligation of the right common carotid artery (CCA) with 4-0 suture silk. A 5 F sheath was inserted in a retrograde manner into the mid portion of the artery where a 2 F Fogarty catheter was placed at the origin of the right common carotid artery at the brachiocephalic take-off. The balloon was inflated for a period of 2 min to ensure cessation of flow in the vessel and then a microcatheter was placed through the sheath and a total of 100 units of prepared porcine elastase were injected into the right common carotid artery for 20 min. The right CCA was then ligated as the sheath was removed and the subcutaneous tissue and incision were closed. The animals were ready for treatment with embolization at day 25±5 days after surgical ligation of the right CCA.
Coil embolization procedure
The femoral artery was exposed and a 6 F Terumo glidesheath was placed after a 1–2 mm bilevel arteriotomy was made. Nitroglycerin was administered to treat vasospasm of the sheath insertion site at a dose of 100 μg when required. A total of 150 units/kg heparin was infused intra-arterially for the procedure. The sheath was placed at the level of the descending aorta under fluoroscopic guidance and the Comaneci device or Hyperglide balloon device was then navigated over a 0.014 inch or 0.010 inch microwire, respectively. A microcatheter was then used to place coils into the aneurysm. Sequential coil embolization was performed with each device being intermittently inflated/deflated for the purpose of the procedure.
After the procedure the animals were killed at either 4±1 days or 28±2 days using standard procedure. The subclavian artery along with the aneurysm was harvested and analyzed using SEM and LM.
Results
A total of 20 rabbits with a mean weight of 3.1±0.2 kg were studied; 12 rabbits were treated with the Comaneci device assisted embolization and eight rabbits were treated with a Hyperglide balloon assisted embolization. Of the Comaneci treated rabbits, six were analyzed in the subacute period (three with LM and three with SEM) and six in the chronic phase (three with LM and three with SEM) while, of the Hyperglide treated rabbits, four were studied in the subacute phase (two with LM and two with SEM) and four in the chronic phase (two with LM and two with SEM).
When analyzing the animals treated in the subacute phase with LM (table 1), medial disruption of the wall was noted in one of the three rabbits treated with the Comaneci device, with the vessel being widely patent. The other two rabbits exhibited no evidence of endothelial injury (figure 2). One of the two rabbits treated with the Hyperglide device studied with LM was noted to have eccentric neointimal growth with mild platelet adherence.
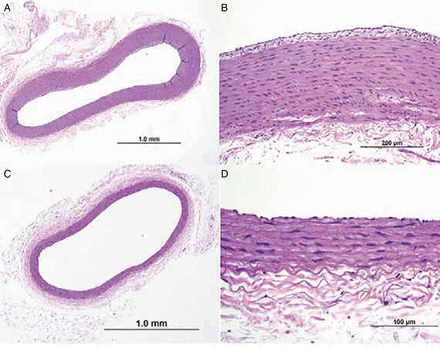
Light microscopy histology findings in rabbits treated with the two devices in the chronic phase
Cross-section of the artery from a rabbit treated with the Comaneci devices proximal (A, B) and distal (C, D). Images A and C show a widely patent lumen and B and D show intact vessel walls without evidence of neoinitmal injury.
In the chronic phase, three of the rabbits treated with the Comaneci device showed no evidence of parent vessel injury under LM and one of the two rabbits treated with the Hyperglide balloon was noted to have smooth muscle loss leading to mild narrowing of the parent vessel. SEM in the chronic phase (table 2) showed that, in one of the three rabbits treated with the Comaneci device, there was a small area of denudation of the endothelium with platelet adherence near the neck of the aneurysm (figure 3). In one rabbit treated with the Hyperglide balloon there was evidence of a denuded endothelium near the neck of the aneurysm with platelet adhesions (figure 4).
Scanning electron microscopy findings in rabbits treated with the two devices in subacute phase
Higher power view of the artery surface outside the aneurysm from a rabbit treated with the Comaneci device. Magnifications 50×, 200×, and 600× sequentially. There is evidence of denuded endothelium with platelet adherence noted near the neck of the aneurysm (red arrow).
Higher power view of the artery surface outside the aneurysm from an animal treated with the Hyperglide balloon. Magnifications 50×, 200×, and 600× sequentially. There is evidence of endothelial damage of the parent lumen with interposed cells and disrupted membranes (red arrows).
Overall, aneurysm obliteration was not different in the two group and the rates of endothelial injury were similar, confirming the safety profile of the Comaneci device.
Discussion
This preclinical study demonstrates the safety of the Comaneci device as a neck bridging device for cerebral aneurysms when compared with the Hyperglide balloon. Importantly, there were no differences in endothelial injury, aneurysmal occlusion rates, and distal embolization between the two groups. The device delivery and tracking were acceptable for both devices.
The use of intracranial stents to support the coiling of a cerebral aneurysm offers advantages and disadvantages. There is a theoretical scaffolding of the stent along the neck that allows for a coil/stent interface that may reduce recanalization rates of the aneurysm. Moreover, the neck of the aneurysm may be integrated as part of a dysplastic vessel in which the stent may provide the construct that will allow healing of the vessel. One group found that patients treated with intracranial stents had a higher aneurysm obliteration rate and lower retreatment rate compared with the balloon remodeling technique.4 The challenge surrounding stenting is the requirement for dual antiplatelet agents which probably leads to an unacceptably high complication rate in the treatment of a ruptured cerebral aneurysm. Patients treated with stents appear to have a higher rate of periprocedural complications (7.4%) and higher mortality than those treated without stents.5 Lastly, there is a risk of delayed ischemic complications due to in-stent restenosis or thrombosis that may increase the morbidity associated with the procedure.6
Balloon remodeling as an adjuvant for the treatment of cerebral aneurysms offers the ability to arrest flow in the case of an aneurysm rupture during coil embolization. This can potentially reduce morbidity, but the use of the balloon itself may lead to a higher rate of aneurysm ruptures during treatment.7 The use of balloons may lead to a higher rate of asymptomatic and symptomatic ischemic lesions on MRI due to flow arrest cause by the inflations.8 Lastly, during deflation of the balloon there is the risk of coil protrusion into the parent vessel.
Self-expandable retrievable stents are currently available to treat cerebral aneurysms in Europe. The current iterations can be partially deployed and then resheathed without deploying the stent (ie, Enterprise Stent, Leo Stent, Solitaire AB). There is a small risk with these technologies of inadvertently deploying the stent by unsheathing too far. Additionally, the current iterations cannot be adjusted for sizing and foreshortening, as can be performed with the Comaneci device. The handle allows the operator to adjust the deployment of the device to conform to the various anatomical variances that may occur during the treatment of cerebral aneurysms. The device is also not designed for permanent deployment, unlike the other technologies that can be used as temporary bridging devices. There are theoretical risks with temporary bridging including a coil being ensnared in the device that may lead to untoward ischemic events. We did not experience this in the 12 animals treated in this study, but care must be taken to ensure the coil is in an adequate position prior to detachment.
Nonetheless, the Comaneci device offers a novel treatment strategy where a mesh delivered through a 0.021 inch microcatheter can be deployed and allow for temporary neck bridging as the aneurysm is coiled. This treatment modality does not require dual antiplatelet agents as the mesh is removed after treatment. In the current preclinical study there does not appear to be any evidence that this technology induces injury to the parent vessel in the subacute or chronic phase.
Footnotes
Contributors RG and RE developed the research hypothesis and wrote the manuscript. FDK and RV assisted with interpretation of results and critical revision of the manuscript.
Competing interests RG: consultant for Stryker Neurovascular, Covidien, and Rapid Medical; Steering Committee for THERAPY trial sponsored by Penumbra and DAWN trial sponsored by Stryker; royalties from UpToDate; Associate Editor for Journal of Neuroimaging and Journal of Neurointerventional Surgery. FDK and RV: Board of directors for CVPath Institute. RE: CEO of Rapid Medical.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Any unpublished data surrounding the individual animal results and histopathologies can be requested by emailing the corresponding author for permission.