Article Text
Abstract
Introduction In acute ischemic stroke (AIS), posterior circulation large vessel occlusions (LVOs) have been associated with poorer outcomes compared with anterior circulation LVOs. The outcomes of anterior versus posterior circulation thrombectomy for LVOs were compared at a high volume center employing a direct aspiration first pass technique (ADAPT).
Methods We retrospectively studied a database of AIS cases that underwent ADAPT thrombectomy for LVOs. Cases were grouped by anatomical location of thrombectomy (posterior vs anterior circulation), and analysis was performed on both entire sample size.
Results A total of 436 AIS patients (50.2% women, mean age 67.3 years) underwent ADAPT thrombectomy for LVO during the study period, of whom 13% of had posterior circulation thrombectomy. Patients with posterior circulation thrombectomy did not show a significant difference in preprocedural variables, including age, baseline National Institutes of Health Stroke Scale (NIHSS), and onset to groin time, compared with anterior circulation (P>0.05). There were also no differences in procedural variables between the two groups. Patients in the posterior group were found to have a similar likelihood of good outcome (modified Rankin Scale score 0—2) at 90 days compared with the anterior group (42.9% vs 43.2%, respectively), and a small but not significant increase in mortality at 90 days. Multilogistic regression analysis showed that the anatomical location (anterior vs posterior) was not an independent predictor of good outcome or mortality after thrombectomy. Prominent predictors of outcome/mortality included age, female gender, procedure time, and baseline NIHSS.
Conclusions Our findings demonstrate that when patients are carefully selected for thrombectomy, those with posterior circulation LVOs can achieve similar outcomes compared with anterior circulation thrombectomy, indicating comparable safety and efficacy profiles.
- Stroke
- Thrombectomy
Statistics from Altmetric.com
Introduction
Endovascular thrombectomy techniques to recanalize a large vessel occlusion (LVO) after acute ischemic stroke (AIS) have become critical components of the standard of care management of AIS patients. Recently completed randomized controlled trials on endovascular thrombolysis and thrombectomy for anterior circulation LVOs have demonstrated a clear clinical benefit over medical management alone.1–11 Endovascular approaches have been shown to reduce the mortality and severity of AIS with an extended window of efficacy compared with intravenous thrombolysis.3–9
Despite the efficacy of current endovascular interventions, outcomes after AIS thrombectomy are still variable.12 Several prognostic variables have been studied to determine major predictors of good outcomes, such as time from onset to treatment, procedure time, baseline deficits, age, comorbidities, and others.13–16 However, the recent randomized controlled trials on AIS thrombectomy have selectively included patients with anterior circulation thrombectomy, with emphasis on middle cerebral artery stroke, and posterior circulation LVO was excluded.1 3 4 6–11 13 17–19 Older studies on posterior circulation LVOs have reported poorer outcomes and higher mortality compared with anterior circulation stroke (ACS).20–24 More recent studies, including the New England Center Posterior Circulation Registry, have shown a more benign outcome for posterior circulation stroke (PCS), and no significant differences in clinical features of PCS compared with ACS.20 25 Due to the lack of level 1 evidence supporting the use of thrombectomy after PCS, the rationale for performing these procedures is predominantly based on evidence from trials involving ACS. However, the differences in outcomes after thrombectomy between the two anatomical subsets have not been compared. Few case series and case reports have demonstrated efficacy and safety of posterior circulation thrombectomy in single arm studies.
Therefore, the purpose of this work was to assess the difference in neurological outcomes, mortality, length of stay, and rates of complications between posterior and anterior circulation thrombectomy at a high volume center.
Methods
Patient selection
We retrospectively studied a database of AIS cases that underwent a direct aspiration first pass technique (ADAPT) thrombectomy at a tertiary care center between January 2013 and June 2017. The study was approved by the institutional review board at the Medical University of South Carolina.
For anterior circulation LVO, patients were included in this study if CT perfusion imaging demonstrated mismatch between cerebral blood volume and blood flow corresponding to regions of penumbra that could significantly contribute to their presenting National Institutes of Health Stroke Scale (NIHSS) score.14 For PCS, less reliance is placed on CT perfusion given the limitations of this modality to assess the posterior fossa and brainstem. Instead, clinical judgment and selective use of MRI to assess for infarct burden to the brainstem were utilized and are described here.
For patients presenting within 10 hours of symptom onset, thrombectomy was attempted. For patients presenting with a prolonged time from onset (>10 hour) or showing clinical deterioration prior to arrival at the treating institution, MRI was obtained prior to intervention. If diffusion weighted image involvement was extensive (ie, more than half of the brainstem demonstrates infarct on any given axial MRI slice) and the patient was not expected to benefit from revascularization attempts, thrombectomy was not offered. All patients were included in this study irrespective of age, time of stroke onset, and whether intravenous tissue plasminogen activator (IV tPA) was administered.
ADAPT thrombectomy was performed as described previously.4 14 The choice of device was based on the largest caliber aspiration catheter that the vessel can accommodate, including 5 MAX, 5 MAX ACE, 064 and 068 catheters (Penumbra, Alameda, California, USA) for M1 or basilar thrombi, and 4 Max or 3 Max catheters (Penumbra) for smaller caliber or more distal vessels. A maximum of 3–4 attempts are performed with ADAPT prior to the use of additional devices, such as stent retrievers, at the discretion of the operator. The majority of patients were subjected to conscious sedation for the procedure while maintain a permissive hypertensive blood pressure to allow collaterals blood flow. General anesthesia was used in 33% of patients in the PCS group and in 6.5% of patients in the ACS group. Following thrombectomy, all patients underwent a postprocedural CT within 12–24 hour, and the presence of hemorrhage on CT was determined and scored by a blinded neuroradiologist.
Data collection
Data reporting preprocedural, procedural, and postprocedural variables were extracted through a retrospective review of patient charts, procedure notes, angiograms, and progress and follow-up notes. Preprocedural variables included in this analysis were age, gender, race, presenting NIHSS, time from symptom onset, comorbidities (diabetes, hypertension, atrial fibrillation, and hyperlipidemia), and whether IV tPA was administered. Procedural variables included the time to achieve recanalization (procedure time), whether intra-arterial (IA) tPA was administered, number of vessels/branches subjected to thrombectomy, the Thrombolysis in Cerebral Ischemia (TICI) Scale, intraprocedural complications, postprocedural hemorrhage, number of devices uses, and number of passes including ADAPT and other devices. Hemorrhage was classified using the ECASS radiological classification as hemorrhagic infarcts (HI1 and HI2), parenchymal hematomas (PH1 and PH2), and subarachnoid hemorrhage.26
Outcomes
Outcomes were assessed using 90 day follow-up modified Rankin Scale (mRS) scores, 90 day NIHSS scores, and length of stay. A good outcome was defined as mRS 0–2 and a poor outcome as mRS 3–6. The 90 day mRS scores and NIHSS scores were collected by a stroke neurologist during routine follow-up visits at 90 days (±14) after stroke for the majority of patients. Telephone encounters with rehabilitation facilities or nursing homes were used to obtain mRS scores for patients in hospice or who died during this interval.
Statistical analysis
Statistical analyses were performed using SPSS V.24 (IBM Corporation, New York, USA) for the majority of the data, and Graphpad Prism 6 (Graphpad, La Jolla, California, USA) was used for analyses in figures 2 and 3. Patient variables were analyzed using descriptive statistics and univariate comparisons. Comparisons were performed using the Student’s t test for continuous measures, non-parametric t test for non-continuous variables, and a χ2 test for categorical measures. The Fisher’s exact test for categorical measures was used when the expected cell sizes were <5. All tests were two sided and an α <0.05 was considered significant.
Following univariate analysis, multivariate logistic regression was used to assess whether location of stroke (anterior vs posterior) can independently predict good outcome (mRS 0—2) or mortality at 90 days after the procedure based on several prespecified prognostic variables, including time to recanalization. Two different models were constructed (for good outcome and for mortality prediction), and the performance of each model was assessed using the Hosmer–Lemeshow test and c statistic. There were 33 subjects with missing values for one or more prognostic values and were excluded from the model. Variables included in these models were: age, gender, race, onset to groin, baseline NIHSS, IV tPA, IA tPA, procedure time, location (anterior vs posterior), comorbidities, complications, a good TICI (≥2B), more than 3 attempts, more than 2 vessels involved, and more than 3 devices used.
Results
Patient baseline characteristics
A total of 536 patients underwent mechanical thrombectomy for AIS during the study period. Patients who did not undergo ADAPT thrombectomy (n=15), and those with no mRS score available at 90 days (±14) were excluded from the analysis (n=85). Therefore, 436 patients (mean age 67.3±14.5 years, 50.2% women, 61% Caucasian) who underwent ADAPT thrombectomy for anterior or posterior AIS during the study period were included in the subsequent analysis. A total of 56 patients underwent posterior circulation thrombectomy involving vertebral, basilar, or posterior cerebral arteries, and 380 patients underwent anterior circulation thrombectomy. Figure 1 illustrates the distribution of thrombi across the different vessels; thrombi occurred most commonly in the middle cerebral artery among ACS and in the basilar artery among PCS patients. The two groups were not different with respect to age, gender, or race (P>0.05) (table 1). The average NIHSS at presentation was 15.3±7 in the anterior group compared with 17.4±11 in the posterior group; however, the difference was not significant (P>0.05) (table 1). A subset of PCS patients (17%) underwent MRI imaging prior to thrombectomy; however, there was no significant difference between the anterior and posterior groups with respect to time from symptom onset to groin (492±812 min vs 480±846 min, respectively). There was also no significant difference on whether IV tPA was administered (40.3% vs 33.9%, respectively). The rates of comorbid diabetes, hypertension, atrial fibrillation, and hyperlipidemia were also not significantly different between the anterior and posterior groups (P>0.05) (table 1).
Distribution of thrombi across the different cerebral vessels in all patients with anterior circulation stroke (ACS) and posterior circulation stroke (PCS). ACA, anterior cerebral artery; Bas, basilar artery; ICA, internal carotid artery; MCA, middle cerebral artery; Vert, vertebral artery; PCA, posterior cerebral artery (P1 segment).
Characteristics of patients categorized by anatomical location of thrombectomy
Procedural variables
The mean time to recanalization was 27.8±23 min in all patients, and was comparable between the anterior and posterior groups (28.1±24 min vs 26.3±20 min, P>0.05). Approximately 17% of patients received IA tPA during the procedure without a significant difference between the groups (table 1). There were also no significant differences in the number of vessels or branches involved in the thrombectomy procedure, number of devices used, or number of reperfusion attempts including non-ADAPT attempts (P>0.05) (table 1). Devices used in the ADAPT procedure were primarily the largest caliber aspiration catheter that the vessel can accommodate, including 3 MAX, 4 MAX, 5 MAX, 5 MAX ACE, and 064 catheters. On failure of ADAPT, additional devices, including stent retrievers, stents, and balloons, were used. There was no significant difference in the number of patients for whom a stent retriever, stent, or balloon was used between the two groups. The percentage of patients with good TICI flow (≥2B) was 92.9% in all patients, and was 92.4% in the anterior group compared with 96.4% in the posterior group (P>0.05).
Procedural complications
Immediate procedural complications were observed in 3.4% of all patients, and the rates of complications were not significantly different between the anterior (3.2%) and posterior (5.4%) groups. One patient developed non-flow limiting internal carotid artery dissection treated with stent placement, another patient developed ST segment depression, and the remaining patients developed contrast extravasation. PH2 specific hemorrhage was observed on postprocedural CT in 5.3%, rates that were comparable between the anterior and posterior groups.
Neurological outcome
Neurological outcome was assessed using the 90 day mRS scores of patients, and was available for 436 patients (figure 2A). The median mRS score across all patient was 3 (IQR 4), which was similar in the anterior (3, IQR 3) and posterior (3, IQR 5) groups (figure 2B). Good outcome was defined as an mRS score of 0–2 and a poor outcome was defined as an mRS score of >2. The rates of good outcome were similar in the anterior group (43.2%) compared with the posterior group (42.9%, P=0.9662) (table 1). The NIHSS at 90 days was only available for 33% of patients, and was not significantly different between the anterior (2.71±4.3) and posterior (1.28±1.45) groups (P>0.05). The overall mortality rate was 19.3% across all groups and was slightly but not significantly higher in the posterior (28.6%) compared with the anterior (17.9%) group (P>0.05) (figure 3). Finally, there was no difference in the length of stay between the two groups, and the overall length of stay was 10±10.3 days (table 1).
(A) Distribution of modified Rankin Scale (mRS) scores among patients within the anterior circulation versus posterior circulation groups. (B) Comparison of mRS scores between anterior and posterior circulation groups, showing no significant difference. Mann–Whitney test, two tailed. Bars denote the range of scores; lines represent median scores.
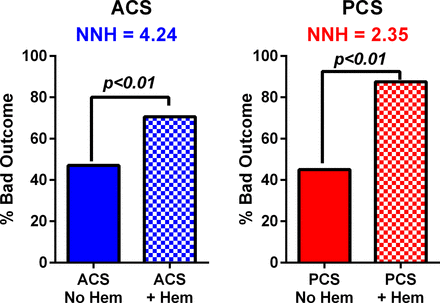
Impact of hemorrhage on 90 day outcomes among anterior circulation stroke (ACS) and posterior circulation stroke (PCS) groups, showing that hemorrhage (hem) significantly increases the risk of a bad outcome in either groups (P<0.01), but the magnitude of effect measured by the number needed to harm (NNH) was higher in the PCS group compared with the ACS group.
Factors predicting outcome
We then assessed, using multilogistic regression analysis, the factors predicting good outcomes after thrombectomy to determine whether the anatomical location (anterior vs posterior circulation) is an independent predictor of good outcomes at 90 days (mRS 0–2). Significant predictors of good outcome included age (OR=0.97, P=0.007), baseline NIHSS (OR=0.87, P<0.001), procedure time (OR=0.99, P=0.033), and the absence of postprocedural hemorrhage (OR=0.39, P=0.001) (table 2). Anterior location of thrombectomy was not an independent predictor of good outcome (P=0.534) (table 2). We further assessed whether the impact of hemorrhage on outcomes was different between the two groups (figure 3). Hemorrhage after PCS thrombectomy was associated with an increased risk of poor outcome compared with hemorrhage after ACS thrombectomy, where the number needed to harm was 2.35 compared with 4.24 in the PCS and ACS groups, respectively.
Results of multivariable logistic regression for predictors of good outcome
Factors predicting mortality
Next we assessed whether the anatomical location (anterior vs posterior circulation) was an independent predictor of mortality at 90 days (mRS 0–2) using multilogistic regression analysis (table 3). Significant predictors of good outcome included age (OR=1.04, P=0.001) and baseline NIHSS (OR=1.09, P<0.001) (table 2). Anterior location of thrombectomy was not an independent predictor of good outcome (P=0.216) (table 2).
Results of the multivariable logistic regression for predictors of mortality
Discussion
Mechanical thrombectomy represents a major advancement in the treatment of a subset of acute stroke patients with LVO, and has consistently demonstrated additional benefits in reducing the severity of neurological deficits after stroke with an extended therapeutic window.3 4 6–11 19 26 However, the major randomized controlled trials on mechanical thrombectomy have only included patients with ACS undergoing the procedure, although thrombectomy can often be performed in patients with PCS.3 4 6–11 19 26 Although ACS is more common than PCS and will thus represent the majority of patients undergoing thrombectomies, there is still limited evidence on whether mechanical thrombectomy has a similar safety and efficacy profile in PCS compared with ACS. In this report, we demonstrated that ACS and PCS thrombectomies using ADAPT were predominantly alike in terms of preprocedural demographics and covariates, variables relating to the thrombectomy procedure, the rate of complications, and most importantly outcomes.
The results of this study demonstrated that among patients carefully selected for candidacy for thrombectomy with CT perfusion (±MRI for posterior LVO), presenting with both anterior and posterior LVOs with similar baseline characteristics, including age, NIHSS at presentation, time from stroke onset to procedure, administration of intravenous thrombolysis, and medical comorbidities, similar rates of good outcome, defined as an mRS score of 0–2 at 90 days, can be achieved. Although there was no significant difference in functional outcomes between the anterior and posterior groups, there was a small, although not significant, increase in mortality in the posterior circulation group compared with the anterior group. There was no difference in procedural variables, rate of complications, rate of hemorrhage, or length of stay between the two groups, suggesting a similar safety profile of thrombectomy in both groups. To verify that the anatomical location (anterior vs posterior) does not contribute to either likelihood of good outcome or mortality after stroke, we performed multivariate logistic regression analysis and showed that anterior location does not significantly predict good outcome or survival after thrombectomy, supporting our initial conclusion. These findings demonstrate that, with careful patient selection, equivalent outcomes following mechanical thrombectomy can be achieved for both anterior and posterior circulation LVOs.
Previous work comparing ACS and PCS presentations and outcomes have demonstrated that ACS and PCS are similar in terms of baseline patient characteristics, etiology of stroke, and outcomes.25 27 Studies that have specifically focused on acute basilar artery occlusion have reported less favorable outcomes in patients with acute basilar occlusion in response to intravenous or intra-arterial thrombolysis due to low recanalization rates (see reviews28 29). The introduction of mechanical thrombectomy for the treatment of vertebrobasilar occlusion resulted in an increase in recanalization rates, decrease in mortality, and increase in the likelihood of good outcomes at 90 days compared with IV or IA thrombolysis.29–35 However, only small studies investigating outcomes after PCS thrombectomies showed favorable outcomes in 20–50% (average 36%)29 32 34 36–40 (reviewed by Möhlenbruch et al 38), which is lower than the rate of favorable outcomes after ACS (33–71%, average 44%).41 Despite this difference across independent studies, comparison of PCS to ACS outcomes after thrombectomy was not performed, and the predominant technique used in PCS thrombectomy was stent retrievers. In this study, we reported on a single, high volume center experience using ADAPT thrombectomy to treat both ACS and PCS while comparing the safety and efficacy profiles. We have demonstrated that the two anatomical subsets may benefit equally from ADAPT thrombectomies in terms of recanalization rates, complications, rates of hemorrhage, and functional outcomes. However, we have shown that postprocedural hemorrhage has a higher negative impact on outcome when it occurs in PCS compared with ACS. Although we did not detect a significant difference in mortality between the two groups with the current sample size, larger multicenter data will be required to further study this effect.
Previous studies in PCS have demonstrated that successful recanalization is the most significant predictor of favorable outcomes.29 38 42 43 Our study showed a high recanalization rate in the PCS groups using ADAPT (96.4% with TICI 2b−3), demonstrating that ADAPT is at least not inferior to stent retrievers (range of TICI 2b–3: 74–100%, average 75%)29 34 38 39 in achieving satisfactory recanalization rates after PCS thrombectomy. The higher rate of successful recanalization of PCS LVOs in our cohort compared with prior studies44 may partially explain the higher rate of good outcomes in this cohort. However, it is pertinent to highlight the significance of appropriate selection of patients for PCS thrombectomy which may better explain the high rate of favorable outcomes. In this study, the candidacy for mechanical thrombectomy was assessed differently in patients with ACS and PCS. Whereas CT perfusion mismatch was predominantly used for assessing the eligibility of ACS patients, CT perfusion is less reliable while assessing infarcts in the posterior fossa or brainstem. Some patients with PCS LVOs were selected based on the use of MRI to assess stroke burden and to direct clinical decision whether to pursue mechanical thrombectomy.
Although our study has shown that the location of the stroke (anterior vs posterior circulation) was not an independent predictor of good outcomes and mortality, several factors were found to affect functional outcomes and mortality after mechanical thrombectomies, including age, female gender, baseline neurological deficits, and duration of procedure. Younger patients and women were the preprocedural variables found to predict good functional outcomes at 90 days (OR=0.98 and 1.76, respectively). The duration of the procedure was another independent predictor of outcome, a finding that supports our previous data on anterior circulation thrombectomy showing that early recanalization time with ADAPT is associated with a higher likelihood of good outcomes.14 15 Here we demonstrate that this relation still holds when ACS and PCS thrombectomies are combined. Finally, baseline NIHSS was a major predictor of both good outcome and mortality, consistent with the stroke literature.45
Limitations
Although the data were obtained from a prospectively maintained database, the retrospective nature of the study is one limitation of this work. Notably, angiographic outcomes were blindly adjudicated and neurological outcomes were assessed by a stroke neurologist not involved in the data collection. The fact that a large number of patients have been enrolled from a single institution in this study minimizes procedural variability and disparities in perioperative patient care. A significant limitation of this work is the low sample size in the PCS group (n=56) patients. The fact that thrombectomy is less commonly performed in PCS compared with ACS patients resulted in fewer numbers of patients in the PCS group. This reduces the power needed to detect changes, especially in mortality between the two groups, where the current study may be under powered. Therefore, follow-up multicenter studies are needed to validate the findings presented in this work.
Conclusion
We have demonstrated that patients presenting with posterior LVOs and AIS benefit equally from thrombectomy with ADAPT compared with those with anterior circulation LVOs. We have described comparable rates of procedural complications and postprocedural hemorrhage, and a similar likelihood of good outcomes. These data suggest that the risk to benefit ratio in PCS thrombectomy is similar to that in ACS, and similar management strategies should be employed. Our study also demonstrated that with careful patient selection and high rates of recanalization, equivalent rates of favorable outcomes can be achieved for posterior circulation LVOs compared with the anterior circulation.
References
Footnotes
Contributors Each author listed above should receive authorship credit based on the material contribution to this article, their revision of this article, and their final approval of this article for submission to this journal.
Competing interests AS: Penumbra-consulting, honorarium, speaker bureau; Pulsar Vascular-consulting, honorarium, speaker bureau; Microvention-consulting, honorarium, speaker bureau, research; Stryker-consulting, honorarium, speaker bureau. AST, RDT, and MIC: Codman-consulting, honorarium, speaker bureau, research funding; Covidien-consulting, honorarium, speaker bureau; Penumbra-consulting, honorarium, speaker bureau, research grants; Microvention-consulting, honorarium, speaker bureau, research grants; Blockade-stock, consulting, honorarium, speaker bureau; Pulsar Vascular-stock, consulting, honorarium, speaker bureau, research; Medtronic-consulting, honorarium, speaker bureau.
Ethics approval The study was approved by the Medical University of South Carolina institutional review board.
Provenance and peer review Not commissioned; externally peer reviewed.