Article Text
Abstract
Background and purpose The aim of our study was to evaluate the safety and efficacy of the pipeline endovascular device for the treatment of anterior circulation aneurysms at the level of the circle of Willis and beyond.
Methods A consecutive series of 25 patients (24 unruptured and one ruptured) with anterior circulation aneurysms treated with a pipeline endovascular device were included in the analysis.
Results We found two minor clinical events (resolved within 7 days of the procedure), one major event (symptoms present after 7 days), and no mortality. There were no aneurysm ruptures or parenchymal hemorrhages during follow-up. The modified Rankin Scale (mRS) scores at 3 and 6 months did not change from the prior mRS score for all cases except 1. There was one asymptomatic periprocedural event. There were three intraprocedural complications which resolved without clinical consequences. Six month follow-up angiograms were obtained for 22 aneurysms, showing complete occlusion in 14 (64%) and significantly decreased residual filling in 8 (36%). The status of branches originating from the aneurysm sacs was evaluated in 14 angiograms: 11 were patent (79%), 2 had moderate reduction (14%) and 1 (7%) was occluded. We found six cases of in-stent stenosis (27%) on 6 month DSA, with only one symptomatic case.
Conclusions The pipeline embolization device provides a feasible and technically safe solution for aneurysms at and beyond the circle of Willis. Preliminary results are promising but larger series with longer term follow-up examinations are required to show the long term safety and durability of this treatment alternative.
- Aneurysm
- Flow Diverter
Statistics from Altmetric.com
Background and purpose
The pipeline embolization device (PED) has become an important tool in the management of intracranial aneurysms.1–3 The device was approved by the Food and Drug Administration in 2011 for the treatment of large and giant wide necked aneurysms arising from the cavernous segment to the superior hypophyseal segment of the internal carotid artery. Although similar periprocedural risks, clinical outcomes, and angiographic results have been described when comparing the PED with stent assisted coiling of anterior circulation aneurysms,4 only a few studies have reported the clinical experience of flow diversion at the level of the circle of Willis.5–7
The aim of our study was to evaluate the safety and efficacy of the PED for the treatment of anterior circulation aneurysms at the level of the circle of Willis and beyond.
Methods
Seven centers (six from Spain and one from the USA) provided retrospective deidentified data on patients with intracranial aneurysms at the level of the circle of Willis and beyond who underwent treatment with PEDs. All centers obtained approval from their institutional review board.
Clinical, procedural, and angiographic data, including aneurysm size and location, PED or PEDs used, initial and last follow-up angiographic occlusion, and clinical follow-up data were analyzed.
In-stent stenosis and all complications were collected and reported. Clinical outcome was defined using the modified Rankin Scale (mRS) score at the scheduled follow-up visit, as assessed and reported by the treatment center.
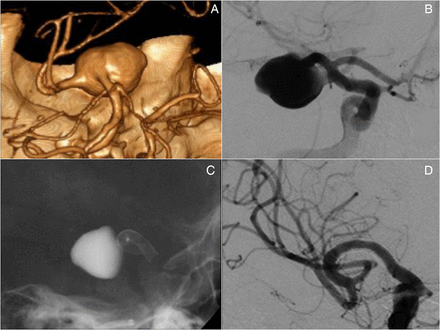
Twenty-five patients (15 women and 10 men; aged 22–79 years; mean age 57 years) with 25 aneurysms were treated with a PED. In 17 cases a bifurcating or distal branch emanated directly from the aneurysm sac (figure 1). All procedures were performed following written informed consent.
Three-dimensional reconstruction and DSA. (A, B) Images show an early frontal branch originating from an M1 saccular aneurysm that was treated with a 2.75×14 mm pipeline embolization device. (C, D) Six month follow-up showing regression of the aneurysm lumen and moderate reduction of the branch.
Aneurysm sizes were classified by their maximum diameter, with an average size of 11 mm (range 1–30 mm).
Twenty-four cases were unruptured. Of these, 15 cases were asymptomatic incidental, 6 cases presented only with headache, 2 patients had seizures only, and 1 patient had headache and seizures. In 3 patients who had PED placement as a retreatment, 3 aneurysms had coils from the previous treatments. The only ruptured case was a blister-like aneurysm at the M1 segment (case No 19).
All patients were premedicated with double antiplatelet therapy (100 mg aspirin in 13 cases, 300 mg in 5 cases, 325 mg in 7 cases, and 75 mg clopidogrel in 24 cases), on average 5 days before the intervention. Platelet function tests were not mandatory and were performed in only 13 cases, with 1 poor responder, who was then given ticagrelor.
All procedures were performed by neurointerventionists with more than 20 cases of PED experience. (In this series, the number of cases per operator were: MM-G 8 cases, GD 5 cases, AR 4 cases, IL 2 cases, PV 2 cases, AV 2 cases, LP 1 case, and JLC 1 case.)
An initial 70–100 U/kg heparin bolus was administered, and activated clotting time was maintained at twice the patient's baseline intraoperatively. Heparin was discontinued but not reversed on conclusion of the procedure. Dual antiplatelet therapy was continued during follow-up.
PEDs were deployed through a Marksman microcatheter (ev3, Irvine, California, USA) using a tri-axial guide–catheter system in all cases.
Any clinical events during the postoperative course were noted. Minor events were considered if symptoms resolved within 7 days and major events if symptoms were present after 7 days. A neurological assessment (mRS score) was performed before treatment, at discharge from hospital, and at follow-up. The protocol included a 24 h postprocedure non-contrast head CT prior to discharge from hospital.
Patients demographics, aneurysm location, type of aneurysm (saccular, dissecting, fusiform), previous treatment, size, rupture status at presentation, degree of occlusion, in-stenosis, patency of side branches whose origin was in the vicinity or was covered by the device, and clinical complications were noted (tables 1, 2).
Demography, aneurysms and devices characteristics, clinical status and follow-up
Demography, aneurysms types, clinical outcome and complications
Aneurysms occlusion was classified as residual aneurysm or occluded (figure 2). The patency of arterial branches was reported as patent, moderate reduction, or occlusion.
(A, B) Three dimensional reconstruction and DSA of a giant aneurysm of the right middle cerebral artery (MCA). (C) Image shows contrast stagnation after pipeline embolization device deployment. (D) Six month DSA showing total occlusion of the aneurysm and patency of the MCA branches.
Results
This series included 25 intracranial aneurysms located at the M1 segment (11 cases), M1–M2 segment (1 case), middle cerebral artery (MCA) bifurcation (6 cases), carotid-T (1 case), anterior communicating artery (1 case), A1–A2 junction (3 cases), pericallosal artery (1 case), and P1–P2 posterior cerebral artery fetal origin (1 case) (tables 1, 2). Sixteen cases were saccular, 5 dissecting, 2 fusiform, 1 saccular–fusiform, and 1 blister-like.
All devices were placed properly, without technical difficulties.
In 16 cases we used 1 PED only, in 2 cases 1 PED and coils (case Nos 8 and 11), in 6 cases 2 PEDs, and in 1 case 4 PEDs.
Postprocedural events
Two minor clinical events (8%) and one major event (4%) occurred, and no mortality (0%) (table 2). The minor event was a mild aphasia that resolved spontaneously 24 h after the procedure (case No 10). The second minor clinical event was a perforator territory ischemia resulting in mild left hemiparesis 2 days after the procedure that resolved spontaneously in 24 h (case No 20). The major clinical event was a disorderly behavior, disoriented in time and space, and with impaired recent memory and disorganized and incoherent speech, secondary to distal multiembolic infarcts in a giant partially thrombosed aneurysm located in the right A1–A2 segment (case No 11). This was treated with a PED and coils through the contralateral A1 (figure 3). The distribution of infarcts were mostly in the anterior cerebral artery distal territory, which can be explained as it was a partially thrombosed giant aneurysm. A right temporal lobe small infarct also occurred that may have been related to the internal carotid artery navigation or to any other intraprocedural maneuver not related to the aneurysm location.
(A) CT angiography, coronal view image, shows a giant partially thrombosed anterior communicating artery aneurysm. (B, C) Three-dimensional/DSA images show a right A1–A2 aneurysm with a jet from the left side. The embolization plan is shown in (B). (D, E) Unsubtracted DSA and roadmap images show pipeline embolization device deployment at the right A1–A2 and coils occluding the left side jet. (F) Postprocedure FLAIR MRI, coronal view, shows small bilateral frontal and right temporal infarcts as complications.
We had 1 asymptomatic periprocedural event, presented as right parenchymal hypodensity in Heubner's artery territory on 24 h control CT prior to discharge from hospital, in the case of a recanalized A1–A2 aneurysm where we used 2 PEDs (case No 5).
Procedural complications
We had three intraprocedural complications which resolved without clinical consequences: one acute branch occlusion during a hypotension episode, which was resolved after increasing arterial pressure (case No 4); one slow opacification of the inferior trunk of the MCA that was covered with the PED, which resolved with an intra-arterial bolus (5 mg) of Reopro (case No 20); and one focal subarachnoid hemorrhage secondary to distal perforation with the microwire during an exchange maneuver as the operator considered that distal support would be necessary in order to navigate the Marksman through the elongated MCA and aneurysm lumen (case No 14). Parent vessel occlusion with coils and glue was done, and then the PED was deployed correctly.
Follow-up
Six-month follow-up angiograms were obtained in 22 of 25 aneurysms, showing complete occlusion in 14 (64%), significantly decreased residual filling in 8 (36%), and PED patency in all cases (figure 4).
Dissecting middle cerebral artery (MCA) aneurysms. (A) DSA shows a dissecting aneurysm located at the right M1 segment. (B) Six month DSA shows a residual lumen and patency of both MCA bifurcation branches. (C) DSA shows another dissecting aneurysm with a saccular component treated with coils in addition to a pipeline embolization device. (D) Six month follow-up shows total occlusion with an asymptomatic in-stent stenosis. In both cases, ipsilateral A1 segments were angiographically occluded asymptomatically at the 6 month follow-up, which could be explained by flow reversal depending on low demand.
Of the 17 cases with branches originating from the sacs, 14 were evaluated with 6 month angiograms: 11 were patent (79%), 2 were moderately reduced (14%), and 1 was occluded (7%). All of these patients with reduced flow or occluded branches were asymptomatic. We found six cases of in-stent stenosis (27%), five of which were asymptomatic.
In the dissecting cases, where the proximal deployment of the device was placed in the distal internal carotid artery, A1 segments were angiographically occluded asymptomatically at the 6 month follow-up (figure 4). Contrast injections from the contralateral internal carotid artery showed flow reversal in both the A2 and A1 segments in all cases.
The only symptomatic stenosis was found at 5 months in the case with 4 PEDs (case No 15) which presented as intermittent mild left-sided weakness that resolved after angioplasty and was stable at 6 months. There were no aneurysm ruptures or parenchymal hemorrhages during follow-up. There were no device migrations during follow-up.
The mRS score at 3 and 6 months did not change from the prior mRS score in all cases except 1 (table 2).
Discussion
The PED (ev3/Covidien Neurovascular) is currently the only Food and Drug Administration approved flow diverter (FD) available in the USA. Recently, the PUFS trial8 demonstrated a high rate of complete occlusion of large and giant wide necked aneurysms of the internal carotid artery and a reasonably low rate of major safety events but to date, only a few series have reported the use of FDs at and beyond the circle of Willis in a limited number of cases.5–7
Chalouhi et al4 noted that flow diversion can be undertaken in small unruptured saccular aneurysms of the anterior circulation, as the PED was associated with similar periprocedural risks, clinical outcomes, and angiographic results compared with stent assisted coiling.
The real incidence of thromboembolic events, occlusion rate, patency of branches, hemorrhagic events, in-stent stenosis, and morbid–mortality of the PED at and beyond the circle of Willis remains unknown, and to our knowledge has only been described in detail in two studies.
Pistocchi et al5 treated 30 aneurysms at and beyond the circle of Willis with FDs (silk and pipeline), reporting transient or reversible ischemic complications in 7.4% of the procedures, permanent neurological complications in only 3.7%, and aneurysm occlusion in 82% of patients. There was no mortality. Lateral branches covered with PED were patent in 38.1%, reduced in 23.8%, and occluded in 38.1% of cases. All cases of flow restriction were clinically silent. Twenty-three of 30 aneurysms (76.7%) were followed-up (2–26 months; mean 13 months): 19 of the 22 aneurysms were treated by FD alone and 4 of the 7 aneurysms were treated by FDs with coils. In the group of aneurysms treated by FDs alone, there were 15/19 complete occlusions (78.9%). In the group of aneurysms treated by FDs and coils, all four aneurysms improved to total occlusion. They encountered 5/23 cases (21.7%) of asymptomatic intra-flow diverter stenosis at follow-up.
Yavuz et al6 treated 25 MCA aneurysms in 21 patients with a PED. The only procedural complication reported was 1 hemorrhagic event (subarachnoid hemorrhage), and the only ischemic periprocedural event was several days after the procedure. They also described two patients with slight left hemiparesthesia 4 weeks after surgery, which resolved with medical treatment in both cases (heparin and steroid therapy, respectively), and one patient who discontinued clopidogrel presented with transient right hemiparesis 3 months after treatment. There was no mortality. Lateral branches covered with PED were patent in 57%, reduced in 28.5%, and occluded asymptomatically in 14% of cases. An interesting point is that one case with reduced branch filling at 6 months recovered its caliber at 18 months, which indicates a hemodynamic reversible effect. In that report, 6 month follow-up angiograms were obtained in 21 aneurysms, showing complete occlusion in 76% and significantly decreased residual filling in 14% of cases. Two aneurysms with residual filling at 6 months showed complete occlusion on 18 month DSA.
In the present study of 25 aneurysms, our results correlate with previous reports. We found two minor clinical events (8%) and one major event (4%), and no mortality (0%). In the subgroup of aneurysms located in A1–A2 (3 cases), different events occurred: one asymptomatic perforator occlusion (case No 5), one symptomatic perforator occlusion (case No 20), and one major event (case No 11). This could be explained by curve related porosity changes of the PED9 ,10 because metal coverage increases in the inner curve of the A1–A2 junction, potentially increasing the risk of perforator occlusion.
We did not find perforator occlusion in MCA aneurysms. This could be explained because perforators at that level originate in a straight segment or at the outer curve.
Based on our results, and on some isolated reports of perforator occlusion,11 we recommend identifying the origin of perforators prior to deployment of the PED, and in case of an origin at the inner curve of the artery, other endovascular or surgical options should be considered.
Six month follow-up angiograms were obtained in 22 aneurysms, showing complete occlusion in 64% and significantly decreased residual filling in 36% of cases. Our understanding, and based on previous reports,6 a side branch which remains patent could delay the total occlusion of the aneurysm until 12 months or more. The status of branches originating from the aneurysm sacs was evaluated in 14 angiograms: 79% were patent, 14% were moderately reduced, and 7% were occluded. All of these patients were asymptomatic.
The status of covered side branches and A1 segments correlated with previous reports where asymptomatic occlusion of branches covered by the device has been described.3 The hypothesis and rational explanations point to hemodynamic regression depending on the demand and collateral supply. This concept needs to be studied and confirmed in long term follow-up studies.
The rate of in-stent in our series (27%) correlated with similar results with other types of intracranial stents described in the literature.12 In our series, no predictive factors could be identified as they were not related to the size of the parent artery, size of the PED, number of PEDs, location of the aneurysm, antiplatelet response status, medication interactions, or any other factors studied. There are no clear recommendations regarding this group of patients with in-stent stenosis, other than continuing with double antiplatelet therapy. Spontaneous resolution has been described with other types of stents after 12–24 months of treatment;12 we will analyze this in the long term follow-up of our series.
Morbidity and mortality rates in our study are in line with previous reports of stent assisted coiling13 ,14 (5.4% rate of thromboembolic events, 6% temporary morbidity, 2.8% permanent morbidity, and 2% mortality) and with recent meta-analysis results15 on FDs (procedure related morbidity and mortality rates of 5% and 4%, respectively).
The major limitations of our case series are the retrospective design and the limited follow-up. The results appear promising but larger series with longer term follow-ups are needed to corroborate the effectiveness of this treatment method and its superiority to other techniques.
Conclusion
The use of the PED beyond the circle of Willis is feasible and technically safe. These preliminary results are promising but larger series with longer term follow-up examinations are required to show the long term safety, efficacy, and durability of this treatment alternative.
Acknowledgments
The authors acknowledge Drs Santiago Pérez, Teresa Díaz, Eduardo Murias, Pedro Ruiz, Eduardo Bárcena, and Eugene Lin for their participation in the procedures.
References
Footnotes
Contributors All authors have contributed to the concept, authorship, and final review of the manuscript.
Competing interests None.
Ethics approval All centers obtained approval for the study from their institutional review board.
Provenance and peer review Not commissioned; externally peer reviewed.