Article Text
Abstract
Background The existence of grey matter (GM) atrophy right after the first clinical event suggestive of multiple sclerosis (MS) remains controversial. The aim of this study was therefore to establish whether regional GM atrophy is already present in the earliest stage of MS assessing regional GM atrophy in a large group of patients.
Methods Sixty-two patients with a clinically isolated syndrome (CIS) were examined on a 1.5 T MR imager within 6 months after their first clinical events. A group of 37 matched healthy control subjects were also included in the study. An optimised voxel-based morphometry (VBM) method customised for MS was applied on volumetric T1-weighted images. The functional status of patients was assessed using the Expanded Disability Status Scale (EDSS) and the Brief Repeatable Battery.
Results VBM analysis (p<0.005, familywise error corrected) on patients versus control subjects showed the presence of significant focal GM atrophy in patients involving the bilateral insula, the bilateral orbitofrontal cortices, the bilateral internal and inferior temporal regions, the posterior cingulate cortex, the bilateral thalami, the bilateral caudate nuclei, the bilateral lenticular nuclei and the bilateral cerebellum. EDSS was slightly correlated (ρ=−0.37 p=0.0027) with the atrophy of the right cerebellum. No correlations have been evidenced between the cognitive status of patients and the regional GM atrophy.
Conclusion The present study performed on a large group of CIS patients demonstrated that regional GM atrophy is present right after the first clinical event of multiple sclerosis and mainly affects the deep GM and the limbic system.
- Multiple sclerosis
- clinically isolated syndrome
- MRI
- atrophy
- voxel based morphometry
Statistics from Altmetric.com
Introduction
Multiple sclerosis (MS) is an inflammatory disease affecting the central nervous system, which is frequently responsible for severe disability, mainly in the form of ambulatory deficits, which often develop after the disease has been evolving for several years. Grey matter (GM) pathology in MS is now well recognised1 and could be relevant to the understanding of the clinico-radiological dissociation in patients with MS, where the focal demyelination in the white matter (WM) observed in conventional MRI cannot fully explain the clinical status (including cognitive impairment).1–3
Various studies based on whole brain or whole GM analyses have shown that particularly high rates of atrophy sometimes occur during the first few years of the disease in patients with a clinical isolated syndrome (CIS) who undergo a subsequent conversion to clinically definite MS, but not in patients in whom this conversion does not occur.4 Two recent studies using voxel-based morphometry (VBM) methods on patients with CIS have yielded contradictory results. In the one study, which involved a relatively small sample of patients (n=28), no regional GM atrophy was detected,5 whereas the authors of the other study, which involved a larger group of patients (n=41), reported the existence of significant regional GM atrophy, mainly located in the deep GM.6
The first aim of the present study was therefore to assess the levels of regional GM atrophy present in a large group of MS patients at the earliest stage of the disease in order to provide evidence for or against the existence of early GM atrophy. The second aim was to study the potential links between regional GM atrophy and clinical status of patients (including cognitive impairment).
Methods
Subjects
Sixty-two patients presenting with CIS and 37 healthy sex–age and educational-level-matched control subjects were included in this study (table 1). All the subjects (patients and controls) were right-handed (>70% Olfield scale) native French speakers. Patients were recruited at the Department of Neurology (University Hospital of Marseille), based on the following criteria: (1) age between 18 and 45; (2) occurrence of the first presumed inflammatory demyelinating event in the central nervous system involving the optic nerve, the spinal cord, a brain hemisphere or the brainstem; (3) no previous history of neurological symptoms suggestive of demyelination; (4) no possible alternative diagnoses (lupus erythematosus, antiphospholipid antibody syndrome, Behçet disease, sarcoidosis, Lyme disease, cerebral arteritis, brain lymphoma, etc); (5) presence of oligoclonal bands on the CSF analysis; (6) presence of two or more lesions in the brain or spinal cord, detected at the initial MRI performed before inclusion. The last two criteria meant that only CIS patients fulfilling at least the dissemination in space criteria according to McDonald7 were recruited. According to these restricted criteria (especially the presence in all the patients of oligoclonal bands in the CSF and at least two T2 white matter (WM) lesions on the brain MRI), the patients included in the present study presented a high risk for developing MS.
Demographic and clinical characteristics of clinically isolated syndrome patients
Patients underwent a clinical examination during the first neurological episode. Another neurological examination was performed on the day of inclusion (the same day when MRI was performed). All the patients' disability levels were rated using the expanded disability status scale (EDSS)8 by the same neurologist (BA) on the day of inclusion. Neuropsychological tests were performed on the patients using the Brief Repeatable Battery (BRB)9 including the Selective Reminding Test, the Spatial Recall Test, the Symbol Digit Modalities Test, the Paced Auditory Serial Addition Task (3′) and the Word list generation.
All the participants gave their informed consent to participating in this study, which was approved by the local Ethics Committee (Timone Hospital, Marseille, France).
Brain MRI
The subjects were examined on a 1.5 T Magnetom Vision Plus MR Imager (Siemens, Erlangen, Germany). A sagittal three-dimensional MP-RAGE T1-weighted sequence (TE/TR=4.7 ms/9.7 ms, flip angle 12°, 128 contiguous slices, matrix=2562, isotropic voxel 1.25 mm×1.25 mm×1.25 mm) and transverse fast double spin echo T2-weighted images (TE1/TE2/TR=15 ms/85 ms/2600 ms, 44 contiguous slices, thickness=3 mm, flip angle=90°, FOV=240 mm, matrix=2562) were acquired on all subjects.
Image processing
WM lesion load
WM lesions visible on T2-weighted images were contoured by the same neurologist (BA) using a semiautomated method (interactive thresholding technique written on the interactive data language (IDL) platform; Research System, Boulder, Colorado, USA).
WM lesion masks labelled as T1-WM lesion masks were identified by simultaneously viewing T1-weighted and T2-weighted images before contouring the lesions on the T1-weighted images using the same semiautomated method (interactive thresholding technique written on the interactive data language (IDL) platform; Research System).
Optimised VBM
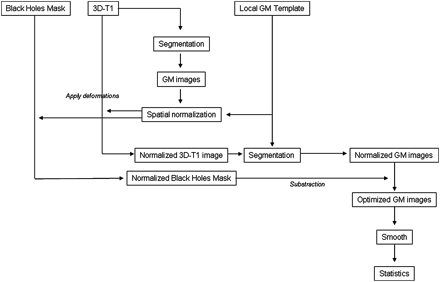
The potential influence of the lesions on the results of the registration is a crucial point in the VBM study performed in MS patients. This potential caveat is probably highly critical in patients with several years of disease evolution when the WM lesion load is important. Various methods have been proposed10 11 to limit this effect. In the present study, to minimise this potential caveat, we used a modified version of the optimised VBM method12 customised for MS,10 where WM lesions masks were applied to patients' scans at the end of images processing to remove any lesional tissue erroneously classified as grey matter. Figure 1 describes the analysis pipeline.
Analysis pipeline of the voxel-based morphometry procedure.
Volumetric T1-weighted images were first normalised spatially (medium regularisation, 7×9×7 nonlinear basis functions) into the MNI space using the T1 anatomical template provided by the SPM2 program. Images were then resampled using an isotropic 1.5 mm×1.5 mm×1.5 mm voxel. The spatial normalisation algorithm preserved the voxel intensities (concentrations) even when region volumes were stretched by warping. After smoothing the images with a 12 mm Gaussian filter, a local T1 template was obtained by averaging the smoothed images obtained with each subject.
Second, the 3D T1-weighted volumes were normalised spatially into the MNI space using the local T1 template previously obtained. Segmentation of these normalised 3D T1-weighted volumes was performed with each subject (SPM2). The resulting normalised GM fraction maps were smoothed using a 12 mm Gaussian filter and averaged across all controls to obtain the locally optimised GM template.
Third, the 3D T1 volumes obtained with each subject were directly segmented. The resulting GM fraction maps were normalised spatially using the locally optimised GM template. The transformation obtained for each spatial normalisation was applied to the 3D T1-weighted volumes and the WM T1 lesion masks of each subject. Then, the normalised 3D T1-weighted volumes were segmented, and the normalised T1 lesion masks were subtracted from the normalised GM fraction maps to prevent misclassification of WM lesion.
Lastly, a conservative threshold of 0.75 was applied to the resulting normalised GM fraction maps free of WM lesions before smoothing the images with a 12 mm FWHM Gaussian kernel.12
Statistical mapping analysis
Between-group comparisons (patients with CIS versus controls) were performed (two-sample t test, p<0.005, k=20, familywise error (FWE) corrected, SPM2) on the smoothed GM fraction maps obtained using the optimised method to determine the location of clusters showing significant differences in the GM concentrations. Coordinates of significant clusters in the MNI space were transformed into Talairach coordinates using a nonlinear transformation to locate these clusters.
Results
Clinical and conventional MRI findings
Patients' demographic and clinical characteristics are given in table 1. The median time between the clinical onset and the inclusion (the time when the MRI was performed) in the study was 4 months (0–6). The median age of the controls was 27 years (20–46), which did not differ significantly from that of the patients (p=0.94). A subpopulation of 37 patients performed the neuropsychological testing. This subpopulation did not differ significantly from the other patients in terms of sex, age, educational level, disease duration, T2 lesion load (T2LL) or EDSS (table 2). We also checked whether there existed any differences in terms of GM atrophy between the two groups of patients (those who underwent the neuropsychological tests and those who did not). These two groups were not found to differ in terms of the regional GM atrophy detected (p<0.005, FWE corrected). The patients' cognitive performances were compared with the performances previously recorded at our laboratory in a group of 52 healthy control subjects. The patients did not differ from this control group in terms of sex, age or educational level (table 2). Patients showed abnormally low performances in the Spatial Recall Test, the Symbol Digit Modalities Test, the Paced Auditory Serial Addition Task (3′) and the Word list generation (table 2).
Neuropsychological tests used and results for healthy controls and clinically isolated syndrome patients
Brain parenchyma fraction (BPF) was not significantly decreased (p=0.06) in patients (BPF=0.831; SD=0.043) compared with controls (BPF=0.847; SD=0.039). GM fraction (GMF) was significantly decreased (p=0.01) in patients (GMF=0.50; SD=0.045) compared with controls (GMF=0.52; SD=0.046). WM fraction (WMF) was not decreased (p=0.94) in patients (WMF=0.33; SD=0.025) compared with controls (WMF=0.33; SD=0.021).
Patterns of regional GM atrophy at the earliest stage of MS
Results are summarised in table 3 and figure 2. At the FWE corrected statistical threshold level of p<0.005, patients showed atrophy localised in the bilateral thalami, the bilateral caudate nuclei, the bilateral lenticular nuclei, the bilateral insula, the bilateral orbitofrontal cortices, the bilateral internal and inferior temporal regions, the posterior cingulate cortex and the bilateral cerebellum, whereas healthy controls showed no significant atrophy compared with patients.
Regions showing significant grey matter atrophy in clinically isolated syndrome patients (n=62) compared with controls (n=37)(p<0.005, FWE corrected)
Regions showing significant grey-matter atrophy in clinically isolated syndrome patients (n=62) compared with controls (n=37) (p<0.005, familywise error corrected). Grey-matter atrophy was located in the bilateral thalami, bilateral caudate nuclei, bilateral lenticular nuclei, bilateral insula, bilateral orbitofrontal cortices, bilateral internal temporal regions, posterior cingulate cortex and bilateral cerebellum.
Correlations between conventional MRI data and regional cerebral atrophy
The correlations have been assessed in the whole group of patients (n=62). The degree of atrophy of the thalami was found to be significantly correlated with the T2LL (right thalamus: ρ=0.57 p=0.001; left thalamus: ρ=0.48 p=0.001). In the whole group of patients (n=62), local GM atrophy was not correlated with global GMF.
Correlations between T2LL, physical and cognitive status
EDSS scores assessed in all the patients (n=62) correlated slightly with the T2LL (ρ=0.26, p=0.03). In the subpopulation of 37 patients with neuropsychological assessment, no correlations were found between T2LL and abnormal neuropsychological performances observed in patients.
Correlations between regional GM atrophy, physical and cognitive status
EDSS scores assessed in all the patients (n=62) were found to be correlated with the degree of atrophy in the right cerebellum (ρ=−0.37 p=0.0027). In the subpopulation of 37 patients having neuropsychological assessment, abnormal neuropsychological performances (Visuo-spatial memory (Short-term recall), Symbol Digit Modalities Test, Paced Auditory Serial Addition Task (3′), Word list generation) did not correlate significantly with the level of GM concentration in regions prone to atrophy.
Discussion
The results of the present study on a large sample of patients with CIS provide evidence that regional GM atrophy is present in the first clinical stage of MS and that it mainly occurs in the deep GM and the limbic system.
Pattern of local GM atrophy in patients in the earliest stage of MS
Atrophy of the deep GM has been well documented in patients with relapsing remitting13 14 and primary progressive10 15 MS. In a previous study on a relatively small group of patients (n=15) with early relapsing remitting MS (RRMS), no regional GM atrophy was detected at inclusion, whereas significant bilateral atrophy of the thalami was present 2 years later.16 Apart form the thalamic atrophy, a subtle involvement of the thalamus may occur in the earliest stage of RRMS6 17: in a study on a small population of patients with CIS (n=18), statistical mapping analysis applied to magnetisation transfer ratio data showed significant tissue matrix disorganisation of the deep GM relative to controls (n=18).17 Recently, a VBM study performed in CIS demonstrated significant regional GM atrophy mainly located in the thalamus.6 In the present study on a larger group of patients with CIS, GM atrophy was detected using the VBM method in the thalamus but also in the large majority of the deep GM structures.
Regional GM atrophy has been previously reported to occur in the temporal and the frontal cortices in patients with RRMS.18 19 In a study using a rather elegant approach to determine the cortical thickness, a local GM thinning was also observed in the cingulate gyrus, insula and associative cortical regions, which correlated with the patients' neurological deficits and their T2 LL scores.20 However, the method used in the latter study did not make it possible to explore the deep GM structures, and no control data were used.20 A recent MRI study on MS patients clearly established that the temporal lobe and the hippocampus were atrophic in patients with MS after several years of evolution.21 Geurts et al22 also detected numerous inflammatory lesions in the hippocampus which may underlie tissue loss and GM atrophy evidenced by MRI.
The pathogenesis of early GM atrophy may involve different processes.23 First, the axonal impairments resulting from WM lesions may induce distal GM lesions secondary to Wallerian degeneration24 or anterograde transynaptic damage.25 26 In a large cohort of patients (n=425) in the advanced stage of the disease, Charil et al observed the existence of correlations between cortical thickness and the total lesion load and disability in the cingulate gyrus, insula and associative cortical regions: these brain regions are strongly interconnected with other brain regions.20 These authors suggested that interruption of WM tracts by MS plaques may contribute to the development of cortical atrophy. Similar observations have been evidenced recently by Henry and colleagues demonstrating a link between WM lesions located in the thalamo-cortical tract and the level of atrophy of the thalami.27 This mechanism may explain the correlations observed in the present study between the T2LL and atrophy of the thalami, one of the most strongly connected GM regions. Another possibility is that a pathological process characterised by iron deposition may be involved in the inflammatory mechanism.28 Significant correlations have been reported to exist between the number of T2 lesions and the abnormal iron deposition rates in the thalami.29 These latter authors suggested that WM lesions may disrupt the axonal iron output, which leads to the accumulation of iron in the deep GM.29
Although the atrophy of the thalami was partly associated with the T2LL, no association with other atrophic GM regions has been evidenced, suggesting that other factors may participate in regional atrophy. First, it is well known that the pathology of the WM is not restricted to the macroscopic WM lesions. Consequently, the mechanisms described above (Wallerian degeneration, anterograde transynaptic damage and disruption of the axonal iron output) may exist in the normal-appearing white matter inducing more diffuse GM atrophy. In addition, the assessment of the potential association between the total lesion load and the regional GM concentration may be suboptimal when considering the possibility of GM damage being mediated through WM tracts.25 Diffusion tensor tractography may be relevant in future studies to better assess the potential link between lesions located in the WM tracts and remote GM pathology. Second, the limited association between WM lesions and GM atrophy may be related to the existence of another pathological process more restricted to the GM.
The authors of several studies have reported the occurrence of inflammatory lesions of the GM in MS. These GM inflammatory processes may consist of focal GM lesions and/or diffuse subpial inflammation.30 The extension of diffuse inflammatory processes in the GM may be more pronounced in patients with progressive forms of the disease.30 Up to now, no evidence has been available as to whether some GM structures may show preferential susceptibility to the GM inflammatory process occurring in MS. One hypothesis is that atrophy—the ultimate consequence of tissue injury—may start in the GM regions which are most sensitive to the diffuse inflammatory process, although this process may not be especially prominent in these regions. Another explanation for the pattern of distribution of the GM atrophy observed is that the GM inflammatory process may predominate in some regions, resulting in significant localised GM atrophy. However, to our knowledge, no data are available so far which shed light on the regional pattern of distribution of the GM inflammatory process in MS.
Another potential mechanism involved in GM atrophy may be represented by the pathology and/or loss of glial cells which represent 60% of the GM cell count in humans.31
Relationships between regional GM atrophy and clinical status
Since the population studied here consisted of patients presented with CIS, the residual EDSS recorded after the relapse was generally low (median 1, range 0–3.5), which meant that few correlations with regional GM atrophy were likely to occur. In addition, the limited association between brain regional GM atrophy and EDSS may be partly due to the characteristics of the EDSS scale particularly sensitive to spinal cord pathology not explored in the present study. Finally, compensatory processes known to occur from the very first stage of the disease onwards may limit the clinical impact of early regional GM loss. The only significant correlation observed, which was between the atrophy of the right cerebellum and the EDSS, was probably due to the fact that the cerebellum contributes importantly to movement control.32
In the present study, the cognitive performances of patients with CIS were significantly impaired in tasks involving working memory, attention and speed of information processing. Previous studies have shown the existence of similar types of cognitive impairment in patients with CIS.33 34 Since all the cognitive abilities in question depend on widely distributed brain networks, these deficits may have resulted from connectivity disturbances secondary to WM injury.35 The lack of correlation observed in the present study between cognitive impairment and regional GM atrophy suggests that the main pathological substrate of cognitive impairment in CIS patients is WM pathology. With the progression of the disease, the contribution of the GM injury probably increases, which would explaining the correlations found to exist between cognitive impairment and GM injury in patients after several years of disease evolution.36 In addition, in a group of patients with RRMS and SPMS, Sanfilipo et al37 observed that WM and GM injury had differential effects on the patients' cognitive performances. WM injury was found to be the best predictor of mental processing speed and working memory, whereas GM injury corresponded to verbal memory, euphoria and disinhibition. In the present study on CIS patients, the fact that the cognitive impairments were restricted to mental processing speed and working memory may explain the lack of correlation observed between the regional GM volume and the cognitive deficits. The characteristics of the cognitive impairments may change slightly with the progression of the disease. In the very first stage, isolated processing speed and working memory deficits are directly related to the state of the WM. After several years, other cognitive abilities such as verbal memory are affected, probably due to the deterioration of the GM.
In addition, in view of the presence of the GM atrophy in the limbic and para-limbic regions, the main functional effect of the early GM pathology may be the emotional disturbances occurring at this stage in the disease. Since the patients' emotions were not assessed in the present study, it was not possible to test this hypothesis.
Conclusion
The present study performed on a large group of CIS patients with very low physical and cognitive disability demonstrated highly significant regional GM atrophy in the deep GM and the limbic system. This study emphasised an involvement of GM by MS pathological process from the onset of the disease.
References
Footnotes
Funding This research was supported by the CNRS, the Institut Universitaire de France, Bayer-Schering France and The French ‘Association pour la Recherche sur la Sclérose en Plaques’ (ARSEP).
Competing interests None.
Patient consent Obtained.
Ethics approval Ethics approval was provided by Timone Hospital, Marseille, France.
Provenance and peer review Not commissioned; externally peer reviewed.