Abstract
Summary: We report a case of pigmented villonodular synovitis involving the temporomandibular joint that presented as a rapidly growing tumor with extension through the skull base into the middle cranial fossa. The case is of interest not only because of the unusual extensive infiltration of this tumor but also because of the role modern diagnostic imaging and endovascular therapeutic techniques played in its diagnosis and management.
Pigmented villonodular synovitis (PVNS) is a tumefactive disease of the synovium of unknown origin (1, 2). It most commonly manifests as a monarticular hyperplastic and inflammatory process involving the large joints of the extremities, with the knee being the most frequently affected site (1–3). PVNS of the temporomandibular joint (TMJ) is uncommon, with 17 cases reported in the literature (4, 5).
We report a case of PVNS of the TMJ that is unique not only because of its unusual aggressive pattern of extensive growth and infiltration but also because of the role modern diagnostic imaging and endovascular therapeutic techniques played in its diagnosis and management.
Case Report
A 37-year-old man was referred to our institution for further diagnostic evaluation and therapeutic planning for a rapidly growing right-sided periauricular mass. Four years earlier, a small painless mass had developed in the same region, and results of an incisional biopsy performed at another institution suggested a diagnosis of brown tumor. The patient was managed expectantly for the next 42 months, at which time he reported rapid enlargement of the mass associated with pain and markedly diminished jaw mobility. There was also subjective and objective right-sided hearing loss (50–60 dB), which prompted a second fine-needle aspiration biopsy, the results of which were suggestive of PVNS. His medical history was significant for chronic renal failure due to chronic glomerulonephritis, for which he had received a kidney transplant.
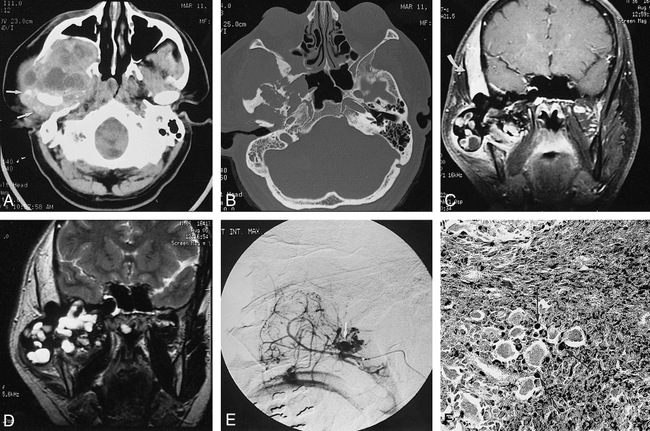
A CT study from another institution showed a large, heterogeneous skull-base mass with an epicenter in the region of the right TMJ. The mass consisted of mixed high- and low-attenuation soft tissue that extended anteriorly into the right infratemporal fossa, inferior orbital fissure, and maxillary sinus, superiorly through the temporal bone into the middle cranial fossa and carotid canal, and inferiorly into the masticator space and parotid gland (Fig 1A). Surrounding the right TMJ were diffuse areas of high attenuation with punctate areas of high density suggestive of hemosiderin deposition and calcification (Fig 1A). There was associated extensive destruction of the skull base and upper mandible (Fig 1B).
37-year-old man with rapidly growing right-sided periauricular mass.
A, Axial CT scan of the skull base shows a large heterogeneous mass in the region of the right TMJ. The tumor has extended into the right infratemporal fossa and bowed the posterior wall of the maxillary sinus anteriorly. Multiple areas of low attenuation are seen, which could represent cysts or necrosis. There is diffuse increased attenuation surrounding the right TMJ with speckled areas of high attenuation, suggestive of hemosiderin deposition and calcification (arrows).
B, Axial CT scan of the skull base (bone windows) shows destruction of the right sphenoid and temporal bones.
C, Contrast-enhanced coronal T1-weighted MR image (700/20/2 [TR/TE/excitations]) shows areas of low signal intensity associated with mostly peripheral enhancement within the large tumor. The tumor has invaded the temporal bone and is slightly compressing the inferior right temporal lobe. There is thickening and enhancement in the right temporalis muscle (arrow).
D, Coronal T2-weighted MR image (2600/100/1) shows blooming of the abnormal low signal intensity within the tumor, consistent with hemosiderin deposition. Multiple areas of focal high signal intensity are seen also, suggestive of cysts or multifocal necrosis. The right temporalis muscle is again noted to be markedly thickened, with diffuse increase in signal.
E, Superselective digital subtraction angiogram of the right internal maxillary artery shows extensive hypervascularity of the tumor with a prominent neovascular blush and focal areas of unusual puddling of contrast material (arrow).
F, Photomicrograph of a surgical pathologic specimen shows extensive iron deposition (black stain) within histiocytes (arrows). Note also the presence of multinucleated giant cells (not staining) and a fibrous background (Prussian blue stain, original magnification ×20).
Subsequent MR imaging performed at our institution before surgery showed the tumor to have heterogeneous signal characteristics on T1- and T2-weighted spin-echo (SE) and fast spoiled-gradient-acquisition (FSPGR) sequences. It measured 9 cm in greatest dimension. The tumor extended intracranially though the floor of the middle fossa, producing mild upward displacement of the right temporal lobe. Extensive areas of peripheral ringlike and central nodular low signal were present on all SE sequences, which showed dramatic blooming on FSPGR sequences (Fig 1C). These findings were consistent with extensive hemosiderin deposition. The right temporalis muscle was markedly enlarged with abnormal, diffuse high signal on T2-weighted SE sequences. The muscle showed diffuse enhancement on administration of contrast material (Fig 1C). The T2-weighted images also showed the tumor to have central areas of high signal with occasional lower signal fluid levels, suggestive of multiple fluid-filled cysts (Fig 1D). Areas of patchy and peripheral enhancement of the tumor were seen upon administration of contrast material. The cross-sectional imaging findings from both CT and MR studies were strongly supportive of a diagnosis of PVNS of the TMJ.
Because of the extent of the tumor and its enhancement pattern, preoperative craniocervical angiography was performed. This study showed that the tumor was hypervascular, deriving most of its supply from numerous branches of the second and third segments of the distal internal maxillary artery (Fig 1E). Extensive neovascularity and prominent tumor staining were associated with unusual puddling of contrast material in portions of the neoplasm.
Since the tumor extended into the right carotid canal, a balloon test occlusion of the right internal carotid artery with hypotensive challenge (6, 7) was performed to help determine the potential risk of intraoperative sacrifice of the artery during resection. Although the patient initially tolerated balloon test occlusion at normotension, left hemiparesis and dysarthria developed after 5 minutes of a provocative pharmacologic hypotensive challenge. The deficits completely resolved shortly after the balloon was deflated.
Because of the extensive neovascularity of the tumor, aggressive transarterial embolization was performed. This involved superselective angiography of the sphenopalatine artery, which was successfully devascularized with a suspension of 150 to 250 μm polyvinyl alcohol particles (Contour, ITC, South San Francisco, CA). Additional tumor supply from the major divisions of the distal internal maxillary artery (deep temporal, accessory meningeal, middle meningeal, and superficial temporal arteries) were each superselectively catheterized and eventually embolized proximally near their origins using various sizes of platinum microcoils (Tornado, Cook, Indianapolis, IN; and Microcoils, Target Therapeutics, Fremont, CA). Postembolization angiography showed good overall devascularization.
Two days later the patient underwent extensive surgical resection of the tumor by a multidisciplinary skull-base team. A modified extended Blair incision was used to preform a total parotidectomy along with resection of the ascending ramus of the mandible, permitting en-bloc removal of a portion of the tumor. A right subtemporal craniotomy was performed to permit resection of the intracranial extent of the tumor. After complete extirpation from the middle cranial fossa, a patch graft of pericranium was used to close the dura. The large resection defect was subsequently covered by a rectus myocutaneous free pedicle flap. The patient fully recovered from surgery and was discharged home 14 days later.
Pathologic examination of the resected specimen showed a friable, darkly pigmented tumor with multiple small cysts on gross inspection. Pathohistologic evaluation showed predominantly plump histiocytes with intermixed, variably distributed, giant cells. The tumor was heavily pigmented with hemosiderin, which was confirmed by iron staining (Fig 1F). There were aggressive histologic features, consisting of invasion and destruction of surrounding bone, but there was no cytologic atypia or mitoses to suggest malignant neoplasia. Frozen sections of the right temporalis muscle showed a focal lymphoplasmacytic infiltrate, with some interposed adipose tissue. However, there was no gross or histologic evidence of PVNS invasion. Occasional areas of chondroid metaplasia with lacy calcification were also seen.
Owing to the unusual nature of the lesion, several pathologists specializing in bone disease reviewed the pathohistologic specimens. The consensus was that the findings represented a very aggressive case of PVNS of the diffuse type, originating from the TMJ.
Discussion
PVNS is a tumorous lesion of the synovium that most often occurs in the knee, hip, shoulder, ankle, and wrist (1, 2); however, any synovial-lined articulation can be affected (2, 3). Although the manifestations of PVNS were probably originally described by Chassiagnac in 1852, Jaffe et al (1) are credited with introducing the currently used term for this disease (1, 5).
PVNS is almost exclusively a monarticular disease, which develops in the second or third decade of life. Usually, this tumor is a slowly growing, nontender swelling of the affected joint. The prevalence of PVNS has been reported to be 1.8 per million population (2).
The pathogenesis of PVNS is unknown (2). Jaffe et al originally thought it was an inflammatory response to an unknown stimulus (1). Others have attributed the process to repetitive intraarticular hemorrhage, trauma, dysfunctional lipid metabolism, or a benign neoplastic proliferation (2, 3, 8). Although PVNS is a tumefactive process, often exhibiting aggressive clinical features, such as local infiltration and osteoinvasion, the disease most likely represents an inflammatory or reactive synovial infiltrate in which the histiocyte plays the predominant role.
On gross examination, PVNS lesions appear as hypertrophied synovium with bunched up masses of villi or nodules, which have been likened to a sponge. The surface is often brown-red to orange-yellow in color (1, 2, 8). Microscopically, the synovium consists of hyperplastic lining cells with rounded masses of fibrous stroma. There is proliferation of plump spindle cells intermixed with giant cells and foamy macrophages containing lipid. Hemosiderin can be found both in the stroma and in the cytoplasm of the synovial lining cells and the macrophages. The yellow coloration is attributed to the foamy macrophages, and the hemosiderin deposits impart the rusty brown color.
The treatment of PVNS of any joint has been predominantly surgical extirpation with complete synovectomy to prevent recurrences (2, 3). Despite these maneuvers, a relatively high rate of recurrence of PVNS has been reported, in the range of 33% to 50% (4). Radiotherapy is often used for treatment of recurrences.
The classic plain radiographic findings of PVNS were described by Smith and Pugh in 1962, and consist of cystic erosions, a lack of joint space narrowing or subchondral osteoporosis, and associated periarticular soft-tissue swelling with no calcification. However, later reports of PVNS have occasionally noted the occurrence of soft-tissue calcification and chondroid metaplasia. Furthermore, in small joints, juxtaarticular cortical erosions and cyst formation are commonly seen. Areas of high density may be seen as a result of the high iron content in the synovium.
With the more recent cross-sectional imaging techniques, better preoperative characterization of PVNS has been possible. For example, CT clearly depicts areas of bone erosion, cyst formation, and extent of the tumor (2, 9). Distinguishing the tumor from surrounding normal tissue is often possible because its attenuation values are usually lower than that of muscle (9) and because of the prominent enhancement of the thickened synovium (8). It has also been noted that areas of PVNS may exhibit high attenuation owing to extensive iron deposition (10, 11).
The appearance of PVNS on MR images is somewhat variable, depending on the specific histologic composition of the tumor (2, 12). Many tumors have areas of characteristic low signal intensity owing to hemosiderin deposition. Hemosiderin, because of its ferromagnetic properties, typically decreases signal intensity on long-TR and long-TE SE sequences (2, 12–14). If the concentration of hemosiderin is high enough, the material may appear dark on all pulse sequences. On T2-weighted SE and GRE images, the abnormal tissue may appear larger because of so-called blooming (2). Areas of high signal intensity on T2-weighted images correspond to a loculated cyst of joint fluid (2, 12). Some authors also have suggested that the accumulation of lipid in foamy macrophages may give rise to areas of high signal intensity on T1-weighted SE images, similar to subcutaneous fat (12). Although MR imaging is best for judging the extent of the tumor (2), it is poor for evaluating the vascularity of such lesions (15).
Conventional angiography has rarely been used for preoperative evaluation of PVNS, and only a few previous reports have described the angiographic appearance of PVNS. Typically, the tumor appears as a highly vascularized mass with arteriovenous shunting, irregular vessels, and dense tumor blush (11, 13, 15). These angiographic features are nonspecific, and are indistinguishable from those of many other benign and malignant neoplasms (13, 15). It has been suggested that as the tumor becomes more fibrotic over time, it may even become hypovascular (11). Despite the few documented observations of hypervascularity associated with PVNS, endovascular therapeutic embolization for palliation or preoperative devascularization has not been previously reported.
PVNS affecting the TMJ is rare, with only 17 cases reported (4). Most patients are female (12/17), and presentation is typically in the third and fourth decades of life (range, 10 to 62 years of age). The most common presenting signs and symptoms are periauricular mass, trismus, malocclusion, and joint pain. Histologically, PVNS of the TMJ typically occurs in the diffuse form, and occasionally exhibits aggressive infiltrative behavior into the skull base, as noted in four of the 17 previously reported cases. As is the case in other anatomic sites, complete surgical resection is the preferred therapy. Of the previously reported cases of PVNS of the TMJ, two recurrences were noted (12%), which were most likely attributable to incomplete resection.
To enhance the likelihood of complete surgical resection of a large and infiltrative tumor of the skull base, detailed and precise localization of the mass is paramount for optimal surgical planning. Clearly, modern cross-sectional imaging techniques play a pivotal role in therapeutic planning. In our case, CT and MR imaging provided important complementary information regarding the extent of destruction of the mandible and skull base in the former and the complex extension of the tumor into various extracranial and intracranial compartments in the latter. Although the temporalis muscle was diffusely enlarged, there was no evidence of tumor invasion. A multidisciplinary surgical team was assembled to effectively handle this complex tumor. As is customary at our institution, the patient was referred for preoperative angiography to evaluate the vascularity of the tumor. We believe that preoperative endovascular therapeutic embolization of such tumors is highly beneficial, because it facilitates complete resection by limiting intraoperative hemorrhage and diminishes the risk of morbidity by improving visualization of important normal anatomic structures within the surgical field (6, 7).
Footnotes
↵1 Address reprint requests to John C. Chaloupka, MD, Interventional Neuroradiology, Department of Diagnostic Radiology, The University of Iowa Hospital and Clinics, 200 Hawkins Dr, Iowa City, IA 52242.
References
- Received November 12, 1997.
- Accepted after revision April 10, 1998.
- Copyright © American Society of Neuroradiology