Abstract
Summary: We present a case of asymptomatic, progressive, late occlusion of the left superior cerebellar artery (SCA) and an aneurysm arising from the junction of the SCA and basilar artery after embolization of an adjacent aneurysm arising between the left posterior cerebral artery and the left SCA. The delayed occlusion was associated with reconfiguration of the Guglielmi detachable coils at the neck of the treated aneurysm.
Postprocedural compaction, reconfiguration, and migration of Guglielmi detachable coils (GDCs) after embolization of intracranial aneurysms is recognized as an important factor in the uncertainty regarding the long-term efficacy of coil embolization of intracranial aneurysms (1, 2). The major series reported to date (2–4) have concentrated on the degree of aneurysmal occlusion obtained with coils, and the subsequent enlargement of neck rests as a consequence of coil compaction or underpacking. Changes of coil configuration within aneurysms that remain completely occluded have not been described. We present a case in which asymptomatic occlusion of an adjacent aneurysm and parent vessel occurred after delayed reconfiguration of the coil mass within the neck of a treated aneurysm.
Case Report
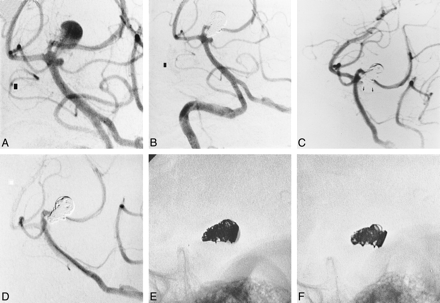
A 61-year-old man presented with a grade-1 subarachnoid hemorrhage on the World Federation of Neurosurgeons Scale. Digital subtraction angiography (DSA) revealed an aneurysm arising from the basilar artery between the left posterior cerebral artery (PCA) and the left superior cerebellar artery (SCA), measuring 9.6 × 6 mm. In addition, a 2-mm-long aneurysm was noted arising from the junction of the basilar artery and left SCA, adjacent to the neck of the larger aneurysm (Fig 1A). Two days later, the larger lesion was embolized using a FasTracker-10 microcatheter (Target Therapeutics, Fremont, CA) and nine GDCs (2D 5 × 15 [3], 4 × 10 [2]), 3 × 10 [2], 2 × 8, 2 × 6). Postembolization DSA (Fig 1B) showed complete occlusion of the aneurysm with preservation of the parent vessels and the small, adjacent aneurysm, which was not treated because of the perceived risk of acute SCA occlusion.
61-year-old man with occlusion of both the left SCA and an aneurysm arising from the junction of the basilar artery and left SCA.
A, Left vertebral arteriogram shows an aneurysm arising from the basilar artery, between the origins of the left PCA and left SCA. A second aneurysm arises from the junction of the basilar artery and left SCA.
B, Right vertebral arteriogram shows complete angiographic occlusion of the larger aneurysm, and preservation of the parent vessels and small, adjacent aneurysm.
C, Left vertebral arteriogram shows complete occlusion of the treated aneurysm, with slow filling of the left SCA (arrows) and adjacent aneurysm.
D, Left vertebral arteriogram shows occlusion of the left SCA and adjacent aneurysm.
E, Lateral view of the coil mass immediately following embolization.
F, Lateral view of the coil mass at 2 years.
DSA at 6 months showed reconfiguration of the coils at the neck of the treated aneurysm, and slowing of flow within the left SCA and the adjacent aneurysm (Fig 1C). The 2-year follow-up DSA showed the left SCA and the adjacent aneurysm were occluded (Fig 1D). Comparison of lateral views of the coil mass immediately after embolization, at 6 months, and at 2 years showed reconfiguration of the coils with significant expansion of the coil mass at the aneurysm neck (Fig 1E and F).
Discussion
One of the major uncertainties related to coil embolization of intracranial aneurysms is the stability of the coils over the medium to long term (1, 2). Aneurysm volume, neck width, angle of arterial inflow, and operator experience have been identified as important predictive factors of the success of aneurysmal occlusion after treatment with detachable coils (5). The best intermediate results have been achieved with small narrow-necked aneurysms (2–4). Initial occlusion of the aneurysm lumen occurs as a result of the combination of the coils and thrombus. Replacement of the thrombus by fibrous tissue is required for permanent occlusion of the aneurysm (6). Stability of thrombus formation has a critical influence on evolution or regression of coiled aneurysms.
Reports of thrombus formation within the coil mass, and subsequent fibrosis and endothelium formation over coils, have had variable conclusions. Stable thrombus formation has been achieved in coiled aneurysms in pigs and dogs (6, 7). Nonetheless, detachable coils did not reliably stimulate pronounced immediate thrombus formation in primates (8), and absence of stable thrombus formation was noted at 6 months in rabbits (9). This last study used a bifurcation model that simulated the flow dynamics within human aneurysms.
In a small number of cases, histopathologic examination of narrow-necked human aneurysms has revealed thick collagenous layers crossing the aneurysm neck, supporting a monolayer of endothelial cells (10, 11). This finding, however, has not been seen in wide-necked aneurysms, which have been shown to remain patent, with incorporation of the coils into a fibrous connective tissue matrix around the periphery of the sac (11, 12). The long-term stability of partially occluded aneurysms remains to be proven irrespective of the width of the aneurysm neck (11).
There are few articles reporting the intermediate and long-term appearances of coiled aneurysms. Byrne et al (2) found that completeness of obliteration improved in 8.5% and deteriorated in 14.7% of aneurysms in their series of 317 patients treated within 30 days of subarachnoid hemorrhage. Late instability of coils is recognized, and delayed migration of coils has been detected at follow-up angiography in at least four cases (13). Three of these did not suffer any neurologic deficit. In two of the cases, reduced flow was noted in the involved parent arteries. Perhaps coil reconfiguration in aneurysms that remain completely occluded on DSA during the follow-up period has not received much attention because of concerns regarding the risks associated with aneurysm recurrence (2–4).
In our case, the coils at the aneurysm neck altered at some point between embolization and the 6-month DSA. Unsubtracted views of the coils at 6 months showed expansion of the coil mass at the aneurysm neck. DSA at 6 months showed slightly delayed flow in the left SCA, and the small aneurysm at the junction of the basilar artery and left SCA was less prominent. The DSA 2 years after the procedure showed no further change in the coil mass, but revealed complete occlusion of both the left SCA and the small aneurysm. Fortunately, the patient was asymptomatic and had no neurologic deficit.
The neck of the coiled aneurysm was intimately related to that of the small aneurysm and, although we felt they were separate lesions, we cannot be certain that they did not represent a single aneurysm with two lobes. We postulate that the late occlusion of the left SCA and small aneurysm may have occurred as a delayed consequence of the reconfiguration of the coil mass. The following are possible mechanisms of the delayed occlusion: 1) mechanical compression caused by reconfiguration and expansion of the coils within the neck of the treated aneurysm, 2) an overactive endothelial response to the coil mass that resulted in gradual occlusion of the origin of the left SCA, 3) spontaneous thrombosis of the left SCA and small aneurysm, or 4) a combination of these factors.
Pathologic reports to date have documented a paucity of endothelial response to detachable coils rather than the endothelial hypertrophy associated with other intravascular prostheses (10–12). There is no angiographic evidence of narrowing of the lumen of the basilar artery or left PCA adjacent to the treated aneurysm; however, the base of the coil mass does appear slightly separated from the lumen of the basilar artery (Fig 1D), possibly supporting the hypothesis of an overactive endothelial response to the coils. Whatever the pathogenetic mechanism in this case, the slowing of flow in the left SCA at 6 months, progressing to occlusion at 2 years, is consistent with a gradual process leading to occlusion. Gradual occlusion also may have facilitated the leptomeningeal collateral supply to the left SCA territory.
Conclusion
To our knowledge, this is the first report of delayed occlusion of a parent vessel and associated aneurysm caused by reconfiguration of the coil mass within the neck of an adjacent intracranial aneurysm. The mechanism of parent vessel occlusion in this case is uncertain, and discussion of the pathogenesis is necessarily speculative. Nonetheless, this case shows that coils within 100%-occluded aneurysms are not necessarily stable. The long-term stability of coils should remain an essential concern in occluded as well as partially occluded aneurysms.
References
- Received December 8, 1999.
- Accepted after revision May 8, 2000.
- Copyright © American Society of Neuroradiology