Abstract
BACKGROUND AND PURPOSE: Scalp swelling associated with cranial burst fracture, a widely diastatic skull fracture of infants associated with dural laceration and acute cerebral extrusion, may be confused with that of a simple subgaleal hematoma. Both conditions can also be associated with hemorrhagic shock. We sought to improve the early evaluation of infants believed to have sustained cranial burst fracture by including MR imaging, since this study clearly delineates the dural-cortical interface, the site of injury.
METHODS: Seven infants aged 1 through 11 months who sustained cranial burst fractures, all initially imaged with skull radiography and CT, were studied or treated from 1992 through 1996. MR imaging was obtained after resuscitation and stabilization.
RESULTS: Surgery or autopsy confirmed MR findings (dural laceration and extracalvarial cerebral tissue) in all seven infants.
CONCLUSION: MR imaging allows early diagnosis of skull fracture associated with acute cerebral extrusion.
Cranial burst fracture, a severe head injury unique to infants, consists of a widely diastatic (> 4 mm) skull fracture associated with acute extracranial cerebral herniation beneath unbroken scalp. At the time of injury or shortly thereafter, these infants often present with seizures, contralateral hemiparesis, a Glasgow Coma Scale (GCS) score of 10 or less, and hemorrhagic shock (1). When scalp swelling associated with cranial burst fracture is attributed to a simple skull fracture and overlying subgaleal hematoma, another condition sometimes accompanied by seizures, lethargy, respiratory failure, and hemorrhagic shock (2), the correct diagnosis is delayed. Weeks to years later, persisting scalp swelling invites further scrutiny after the cranial burst fracture has become a “growing skull fracture” with ongoing neurologic insult, enlarging calvarial and dural defects, and adherence of injured cortex to adjacent skull and overlying soft tissues (3–5).
Because prompt repair of burst fractures may prevent further brain dehiscence and ongoing neurologic injury, acute neuroimaging should delineate the injury: dural laceration and extrusion of cerebral cortex. Although skull radiographs obtained shortly after injury usually show fractures measuring at least 4 mm in width (1, 6–15), and CT may display extruded cortex associated with a diastatic fracture and overlying soft-tissue swelling, skull radiographs can be misleading (13), and CT studies may not always differentiate herniated cortex from scalp edema or subgaleal hematoma. Moreover, it is the presence of dural laceration, poorly delineated on CT scans, that determines whether a diastatic fracture will become a growing skull fracture (3). Descriptions of the MR appearance of growing skull fracture exist, but not in the context of prevention (16–19).
Methods
Seven infants aged 1 to 17 months (five girls and two boys; average age, 4.1 months) with cranial burst fracture were treated or studied from January 1992 through January 1996 (Table). One was involved in a motor vehicle accident, one fell two stories, and five suffered unwitnessed or nonaccidental trauma. All presented with marked scalp swelling and a GCS score of 10 or less. Four manifested clinical seizure activity and five a contralateral hemiparesis (Table).
Clinical data, imaging characteristics, and anatomic findings in seven infants with cranial burst fracture
All seven infants were examined with skull radiography and CT within hours of presentation. Anteroposterior and lateral skull radiographs revealed fractures 5 to 15 mm wide (Fig 1A). MR imaging was performed within 5 days of injury. Multiplanar unenhanced MR studies, consisting of T1-weighted (400–600/20 [TR/TE]) and T2-weighted (2500–3000/80–120) sequences, were obtained with a 1.5-T Signa magnet.
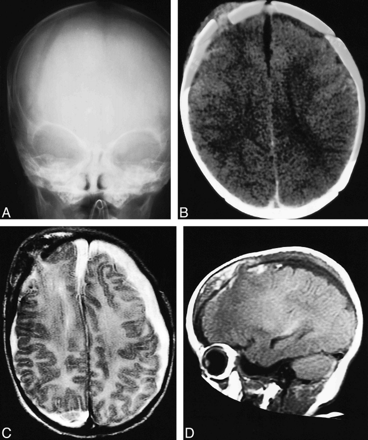
Patient 1.
A, Skull radiograph 4 hours after injury shows a widely diastatic right frontal skull fracture.
B, CT scan of acute cranial burst fracture 4 hours after injury shows hemorrhagic contusion beneath the fracture, overlying scalp swelling, and moderate eversion of fracture edges.
C and D, Axial T2-weighted (3000/100/1) (C ) and sagittal T1-weighted (549/20) (D) MR images 15 hours after injury show acute transcalvarial brain herniation. Brain parenchyma is clearly differentiated from scalp soft tissue and hematoma. Note associated convexity and interhemispheric subdural hematoma and cortical contusions.
Results
CT scans always showed marked soft-tissue swelling over the fracture. Underlying cortical contusion or hemorrhage was present in six infants, usually beneath the fracture, one edge of which was mildly depressed. Associated subdural, subarachnoid, or intraparenchymal hemorrhage was common (Figs 1B, 2A, and 3A). Ventricular displacement toward the fracture, suggesting cerebral extrusion, was noted in two infants, one of whom died. A contralateral subdural hematoma was present in the surviving infant.
MR imaging revealed dural defects and cerebral herniation, confirmed at surgery in all six infants who underwent surgery, and at autopsy in one. T1-weighted images typically showed edematous, mixed-signal hemorrhagic cortex traversing dural and calvarial defects. T2-weighted studies revealed the extent of underlying white matter edema (Figs 1C, 2B, and 3B). Proton density–weighted studies showed herniated cortical vessels especially well. Coronal and sagittal images clearly delineated vertex disorders. Although illustrating cortical contusion beneath the fractures in virtually every patient, CT scans neither differentiated herniated cortex from scalp or subgaleal hematoma nor showed dural tears; ipsilateral ventricular displacement suggested these conditions in two children.
Surgery was delayed until hemodynamic stability and control of intracranial pressure were assured: 1 to 15 days after injury (average, 6.4 days). Herniated cortex is separated from the dural edges, skull, and surrounding soft tissue, allowing primary closure of dural and calvarial defects (Fig 4). One infant requiring urgent evacuation of an expanding intracerebral hematoma underwent repair of the cranial burst fracture on the day of injury. Another, presenting with hemodynamic instability requiring resuscitation en route to the hospital, hematocrit of 5%, and disseminated intravascular coagulopathy, died within 48 hours of hospital admission.
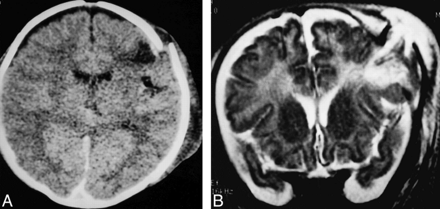
Patient 3.
A, Axial CT scan 3 hours after injury shows diastatic fracture with everted fracture edge and marked scalp swelling. Underlying subdural and subarachnoid hemorrhage and small cortical contusion are present.
B, Coronal T2-weighted (3500/108/1) MR image 36 hours after injury reveals extent of cerebral herniation and dural defect. Note high signal intensity of both intracranial and extracranial brain tissue.
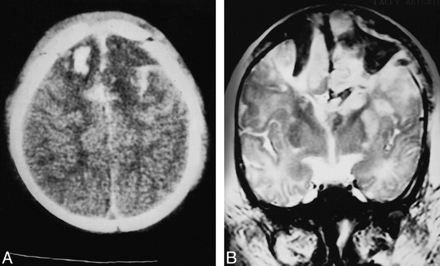
Patient 6.
A, CT scan of fatal cranial burst fracture 2 hours after injury shows diastasis of coronal suture, bifrontal cortical contusions, and marked, high-density scalp swelling.
B, Coronal T2-weighted (3000/108/1) MR image 12 hours after injury reveals transdural herniation of hemorrhagic brain and cortical vessels with underlying cortical contusions.
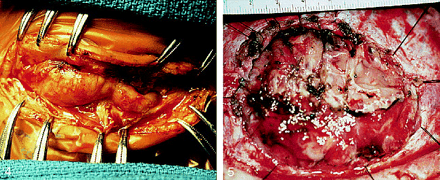
Intraoperative photograph of patient 1. The cerebral cortex is encountered in the subgaleal plane after scalp incision.fig 5. Operative appearance of growing skull fracture that remained untreated for 6 months after injury. A sizable defect has developed, necessitating large dural and split-thickness calvarial grafts. Acutely treated cranial burst fractures rarely require grafts of either skull or dura
Discussion
Cranial burst fracture (1) is a widely diastatic skull fracture associated with dural laceration and extrusion of cerebral tissue outside the calvaria beneath an unbroken scalp. Prompt reduction of the cerebral hernia followed by dural repair prevents a growing skull fracture (3, 5–8, 10, 14, 15, 20–23). Diagnostic strategies have ranged from serial physical examinations in search of an enlarging skull mass to neuroimaging, including plain radiography (7), cerebral arteriography (14, 15), CT (17, 21, 22), color Doppler sonography (24), and MR imaging (25).
Although the overlying scalp usually remains unbroken (10), crushing forces causing significant skull deformation may tear or burst adherent dura mater and extrude cerebral cortex (3, 10, 26, 27). The mechanism of injury appears analogous to the “nutcracker” injury sustained by infants suffering air bag–related trauma (28). Elevated intracranial pressure resulting from cerebral swelling, hemorrhage, or hydrocephalus in the presence of a dural tear can also facilitate cerebral dehiscence.
Growing skull fractures can develop when dural and arachnoid tears remain unrepaired (27, 29, 30). Goldstein et al (30) demonstrated chronic cerebral herniation into craniectomy defects in experimental animals, and noted that an associated dural defect could prevent or delay fracture healing. Cranial and dural defects associated with arachnoid tears enlarged with or without surgically induced focal brain injury or ventricular disruption (29, 30). Stein and Tenner (14, 15) considered herniated cerebral tissue within the fracture defect the defining feature of growing skull fracture. Lende and Erickson (10) noted that a “dural defect … appears to be a requisite for enlargement of the fracture,” and described underlying cortical injury, often clinically manifested by contralateral hemiparesis and seizures. These authors acknowledged that cerebral swelling might enlarge the defect. Likewise, Penfield (31) proposed that the interposition of cortical tissue between fracture edges accounts for fracture growth. Expanding calvarial defects not associated with underlying cortical injury in patients reoperated for craniosynostosis have been reported and are believed to be the result of unrecognized dural tears sustained during the initial operation (32, 33). Such controlled injuries are not analogous to the massive trauma sustained by our patients.
Neuropathologic findings associated with growing skull fractures may simply represent the static aftermath of severe traumatic brain injury (9). Cerebral changes associated with some growing skull fractures, however, could represent secondary changes arising in the wake of untreated cerebral extrusion (20, 22, 27, 31). Stein and Tenner (14, 15) stated that cortical ischemia (suggested by slow flow at angiography) might cause ongoing neurologic injury in patients suffering cortical herniation associated with their diastatic fractures. Roy et al (27), noting areas of acute and chronic cortical necrosis associated with growing skull fractures long after injury, stated that, “varying degrees of brain damage continue to occur in the area of the main defect and at its edges long after the incidence of the trauma,” and that, “in addition to the damage including laceration of the brain at the time of initial injury, further damage to these structures continues to occur” as evidenced by “the accumulation of compound granular corpuscles and foci of reactive astrocytic cell proliferation.” Consequences of such ongoing injury may transcend local effects. Cytokine production by reactive astrocytes and mononuclear phagocytes occurs months after experimental head injury and may play a role in posttraumatic cerebral degeneration (34).
Ramamurthi and Kalyanaraman (35) questioned the need for repair of growing skull fractures in older patients, yet two of their four subjects manifested progressive neurologic deficits throughout the observation period. Roy et al (27) and Tandon et al (22) reported 60 patients with growing skull fractures, most presenting months to years after injury, two thirds suffering hemiparesis, and nearly one half seizures. More than 10% had progressive neurologic deficits. Others have documented such deterioration (8, 35–37), possibly due to ongoing cerebral damage after the initial injury analogous to that seen in the “syndrome of the trephined” (20, 22, 27, 37). Development of a posttraumatic pseudoaneurysm adjacent to an untreated growing fracture has been reported (12).
Enlargement of cranial defects and cerebral adhesion to overlying scalp unnecessarily complicate repair. Cortical parasitization of scalp vessels occurs as herniated cortex adheres to adjacent tissues (27). Figure 5 shows the operative findings of a cranial burst fracture left untreated for 6 months.
Other injuries must not be confused with cranial burst fracture. Sekhar and Scarff (13) reported two infants with “pseudogrowing skull fractures” after a relatively trivial injury, unaccompanied by neurologic deficit, who never displayed clinical evidence of increased intracranial pressure. These initially minimally diastatic (2-mm wide) fractures had enlarged 2 to 3 mm 3 to 6 weeks later, but went on to heal spontaneously by 18 to 23 weeks.
Blunt impact to an infant's head sufficient to fracture skull and tear or burst dura may be associated with meager clinical findings at the time of injury. Scalp swelling and anemia (26) may be attributed to subgaleal hematoma and soft-tissue swelling alone (1, 3–15, 17, 18, 20–23, 26, 27, 29, 32, 35, 38–44). A close look at the history (severe trauma) and careful physical examination (enlarging scalp mass) may identify growing skull fractures as early as 4 weeks after injury (8, 17, 29, 30), but alone cannot establish the presence of cranial burst fracture soon after the event.
Cerebral angiography aside (15), imaging techniques have elucidated cerebral herniation well after the fact. Although acute skull radiographs do show fracture diastasis exceeding 4 mm, Goldstein et al (30) showed experimentally that bony rarefaction, evidence that dural laceration with dehiscence of brain or meninges or both have occurred, is not identifiable until 6 weeks later. Lye et al (36) used CT to demonstrate subgaleal CSF and soft tissue, presumably herniated cortex, overlying a growing fracture 8 weeks after injury in a patient with a clinically obvious growing skull fracture associated with a fluctuant scalp mass despite a reportedly normal CT study obtained 2 weeks after injury. Nalls et al (21) identified cerebral herniation in a 12-month-old child from unenhanced and enhanced CT studies obtained 10 months after injury, when the nature of the cranial defect was already clinically apparent. Other published reports of CT findings in growing skull fractures are limited to fracture characteristics months to years after injury (22, 38, 45).
Although it remains the preferred imaging technique for acute head injury, CT can fail to show the severity of craniocerebral trauma (42, 46, 47). Inappropriate algorithms, artifacts, and partial volume averaging may obscure injuries located near the vertex or skull base. Unequivocal demonstration of cranial burst fracture on initial CT studies represents the exception. The CT appearance may be deceptively benign, because the fracture margins do not appear greatly depressed and the cortical contusion beneath the fracture may appear trivial (48) (Fig 2A). Although a dedicated pediatric head algorithm optimizes CT detection of acute superficial contusions and focal subarachnoid hemorrhage (findings present in the majority of infants with surgically confirmed cranial burst fracture), differentiating between scalp hematoma and cerebral extrusion by means of CT remains difficult. MR imaging delineates the interface between calvaria and intracranial tissues, the locus of injury in cranial burst fracture, revealing dural defects and acute cerebral extrusion through the calvaria, however subtle (Figs 1–3). Flow voids reveal extracranial cortical vessels. MR imaging surpasses CT in depicting associated intracranial injuries (ie, diffuse axonal injury, small superficial cerebral contusions, small extraaxial collections, primary brain stem injury, and subacute and chronic changes) ensuing after head injury (18, 42, 46, 47, 49). MR imaging defines the age of cerebral injury, an important consideration in cases of suspected nonaccidental trauma (50), a frequent cause of cranial burst fracture in our patients. MR imaging is considered the ideal study of nonaccidental head injury in infants (48, 49). Although Kelly et al (46), in their review of 100 patients with head injury undergoing both CT and MR imaging days to weeks after injury, stated that acute surgical management was not altered by the additional information garnered by MR studies, they acknowledged the latter's usefulness in the subacute or chronic head injury setting as well as in “selected patients.” MR imaging is useful for the prompt identification of infants with cranial burst fracture (25), a condition requiring, in our opinion, timely surgical repair (25).
Conclusion
Cranial burst fracture, a widely diastatic skull fracture with acute cerebral extrusion into the subgaleal space, is a severe traumatic injury of infants. Weeks must pass before physical examination can differentiate a persisting or pulsatile scalp mass from resolving subgaleal hematoma. Skull radiographs confirm the condition only after chronic bony changes have occurred, and CT may not reveal the condition until it is already clinically evident (ie, after chronic neuropathologic and cicatricial changes have occurred). Identification and repair of cranial burst fracture before the chronic neuropathologic changes of growing fracture develop minimizes ongoing cerebral injury and facilitates safe repair. Because it differentiates brain parenchyma from adjacent soft-tissue hematoma and subcutaneous tissue, demonstrating the dural defect and acute subgaleal cerebral extrusion, MR imaging prospectively shows which injury (ie, cranial burst fracture) is likely to become a growing skull fracture, allowing timely repair of dural and calvarial defects after the patient is hemodynamically stable and acute cerebral edema has subsided.
Infants presenting with marked scalp swelling, a GCS score suggesting moderate-to-severe head injury, an acute skull fracture wider than 4 mm on radiographic examination or CT lateral digital radiographs, and subarachnoid or parenchymal blood seen beneath the fracture on CT scans should undergo MR examination after they are clinically stable to identify dural defects and cerebral dehiscence. Coexisting severe scalp swelling and suspected abuse (five of the seven infants reported herein sustained nonaccidental trauma) should also suggest cranial burst fracture. Reduction of herniated neural tissue, dural repair, and skull reconstruction are best performed after acute cerebral swelling has subsided but before cortical parasitization of scalp vessels and scarring have occurred (1, 4, 11, 20, 27, 44). Identification of cranial burst fracture before the chronic neuropathologic changes of growing fracture develop facilitates safe repair and may minimize ongoing cerebral injury.
Footnotes
↵1 Address reprint requests to David J. Donahue, MD, 800 8th Ave, Suite 220, Fort Worth, TX 76104.
References
- Received April 1, 1999.
- Copyright © American Society of Neuroradiology