Abstract
BACKGROUND AND PURPOSE: Bipolar affective disorder (BPAD) can have its onset during childhood, but the diagnosis may be difficult to establish on the basis of clinical findings alone. Our purpose was to determine whether proton MR spectroscopy can be used to identify abnormalities in the brain of children with BPAD.
METHODS: Ten children, ages 6 to 12 years, underwent clinical testing to establish the diagnosis of BPAD. After a drug washout period, all patients underwent MR spectroscopy in which a TE of 135 was used along with a single-voxel placement in both frontal and temporal lobes during a single session. Peaks from N-acetylaspartate (NAA), choline (Cho), glutamate/glutamine (Glu/Gln), and lipids were normalized with respect to the creatine (Cr) peak to obtain ratios of values of peak areas. These data were compared with those obtained in 10 non–age-matched control subjects. To corroborate our data, five children with BPAD also underwent 2D MR spectroscopic studies of the frontal lobes with parameters similar to those used in the single-volume studies.
RESULTS: All children with BPAD had elevated levels of Glu/Gln in both frontal lobes and basal ganglia relative to the control group. Children with BPAD had elevated lipid levels in the frontal lobes but not in the temporal lobes. Levels of NAA and Cho were similar for all locations in both groups. Two-dimensional MR spectroscopic studies in five children with BPAD confirmed the presence of elevated lipids in the frontal lobes.
CONCLUSION: Our preliminary observations suggest that MR spectroscopy may show abnormalities in children with BPAD not found in unaffected control subjects. It remains to be established whether these abnormalities are a signature of the disease and can be used as a screening test.
Bipolar affective disorder (BPAD) is a severe, chronic, and incapacitating illness that may begin during childhood (1). Its course may be nonepisodic and often manifests as a mixed state with both manic and depressive symptoms, which may cycle rapidly. There is a high rate of associated disorders, including anxiety, attention deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorders in children with BPAD, thus making the diagnosis of BPAD difficult to establish in many patients (1). The impact of this illness on the developing brain is not well understood.
Studies of adults in whom affective illnesses develop after brain injuries have found associated abnormalities in the frontal or temporal lobes (2). Mania may be related to right frontotemporal or left parietooccipital lesions and depression to left frontotemporal and right parietooccipital lesions. Imaging findings in these patients are nonspecific, with the most commonly observed abnormality being atrophy (3, 4).
Proton MR spectroscopy provides information regarding tissue biochemistry and metabolic changes in vivo. MR spectroscopy has been used with some success in psychiatric illnesses limited to understanding some metabolic changes and to assessing the effects of lithium in the treatment of BPAD (3–6). Our purpose was to determine whether metabolic abnormalities in the brain of children with BPAD may be detected by MR spectroscopy and to compare MR spectroscopic findings with those obtained in unaffected children.
Methods
We studied 10 children, nine boys and one girl, 6 to 12 years old (mean age, 8 years), who met the Diagnostic and Statistical Manual, 4th ed. (DSM-IV) criteria for BPAD. Medications were withheld for a period of 1 week before the MR spectroscopic examinations. Comorbid diagnoses were present in 88% of the subjects. All children had full-scale IQ levels above 80 and had no history of traumatic brain injury, seizures, or neurologic disorders. Control subjects consisted of 10 non–age-matched children (eight boys and two girls) who did not have psychiatric or neurologic disorders and who were being studied for other reasons; they did not undergo neuropsychological testing. These control subjects were also used in other ongoing studies at our institution. Our institutional review board did not authorize the use of a second group of age-matched control subjects for the present study.
All parents completed the Children's Schedule for Affective Disorders and Schizophrenia (K-SADS), the Mania Rating Scale, and a structured family history. In all cases, the diagnosis of BPAD was based on the children's fulfillment of the DSM-IV criteria and on parents' responses on the K-SADS. The Woodcock-Johnson Psychoeducational Battery-Revised (DML Teaching Resources, Allen, TX) was used to measure selective neurocognitive and academic skills. The NEPSY (The Psychological Corp, San Antonio, TX) test (NEPSY includes a comprehensive neuropsychological battery comprising tasks requiring attention and executive functions, sensorimotor abilities, language, visuospatial functions, and memory) was used to provide a description of the neuropsychological functioning of the children. The Woodcock-Johnson Psychoeducational Battery-Revised was used to obtain estimates of academic achievement and overall intellectual functioning by measuring cognition and achievement and by providing an overall cognitive abilities score as well as specific achievement scores in reading, mathematics, written language, and general knowledge. The mania rating scale was completed by the parents and separately by one examiner.
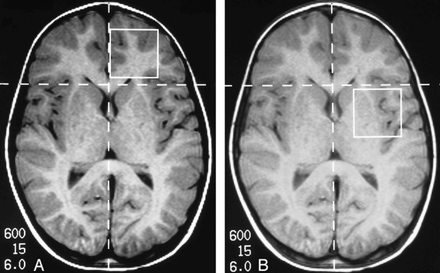
Subjects were scheduled for MR spectroscopy after a drug washout period of 1 week. One child required mild sedation, obtained with 25 mg of diphenhydramine hydrochloride (Benadryl) given intravenously. All patients had undergone past (within 3 months of the MR spectroscopic examination) contrast-enhanced MR imaging studies of the brain, which had been interpreted as normal. Patients were placed in a standard receive-only head coil in a 1.5-T MR system. A set of axial T1-weighted images (750/15/1 [TR/TE/excitations]) was obtained using 5-mm-thick sections as localizers. In 10 patients, a voxel measuring 8 or 27 cm3 (2 × 2 × 2 cm3 or 3 × 3 × 3) was initially placed in the left and right frontal lobes with its medial margin at the anterior interhemispheric fissure and its posterior margin at the frontal horn of the lateral ventricle. This voxel encompassed cortex and white matter (Fig 1A). Voxels of identical dimensions were placed in the left and right temporal lobes, centered in the region of the external capsule and containing insular cortex, deep gray matter structures, and white matter tracts (Fig 1B). These voxels were localized using point-resolved spectroscopy (PRESS) after suppression of the water peak using a chemical-shift selective excitation (7). Studies were obtained with parameters of 1500/135/128 to collect data from these regions. The time domain data collected were processed using a 5- to 8-Hz gaussian filter zero-filled to 2048 and Fourier transformed to obtain the frequency spectra. The spectra were then phase- and baseline-corrected using a second-order spline function. The evaluation was repeated in five patients (also off medication) with a 2D MR spectroscopic technique. The grid was placed encompassing from frontal lobes. The studies were obtained by using PRESS localization and a TE of 135. The size of the individual voxels was 1 × 1 × 2 cm. Each voxel was manually phase- and shift-corrected. Statistical analysis was not performed for the data obtainedfrom this group. Rather, we used the information obtained from these studies as an internal control to eliminate the possibility of contamination by extraneous lipids.
A and B, Axial T1-weighted images show placement of voxel in the left frontal lobe (A) and in the left temporal lobe (B)
Using the curve-fitting software provided by the manufacturer, we determined the peak areas in the single-volume spectra by assuming a gaussian shape for all resonances. We assigned choline (Cho) at 3.2 ppm, creatine (Cr) at 3.03 ppm, and N-acetylaspartate (NAA) at 2.0 ppm (8). The resonances seen between 2.1 and 2.5 ppm were assigned primarily to glutamate/glutamine (Glu/Gln) and those between 0.8 and 1.8 ppm to the methylene and methyl groups of lipids. All resonances were normalized with respect to the Cr peak to obtain ratios. The ratios obtained for each of the metabolites in the four voxels sampled in each patient were then compared with results obtained from the control subjects. All measurements were obtained in a single session. Means and SDs were calculated for NAA/Cr, Cho/Cr, Glu/Gln to Cr, and lipids to Cr.
Results
NEPSY domain scores for our patients were in the low-average to average range. The visuospatial domain was in the average range, with both visuospatial and visuoconstructive abilities equally developed within this range. The language domain was in the average range, with speed naming and phonological processing in the average range and comprehension of instructions in the low-average to average range. Domains of attention/executive, sensorimotor, and memory were low, falling in the low-average range. Our patients had greater difficulty with tasks requiring problem solving, short-term memory for faces and names, visual attention, and visual-motor speed and accuracy. Overall cognitive abilities were in the low-average to average range. Tasks requiring visual attention and short-term visual memory were low. With respect to core academic achievement skills, scores were average for reading, mathematics, and general knowledge. Writing capabilities were in the low-average range, and there was good letter-word identification and reading comprehension, intact basic calculation skills, and a satisfactory understanding of mathematical concepts. In the writing domain, spelling was adequate but patients showed significant problems with word usage, punctuation, and capitalization. While some of these problems may have been influenced by relatively poor memory capabilities, it is also likely that these difficulties were related to inaccessibility to the learning/academic environment, precipitated by their BPAD. Results of the neuropsychological testing are shown in Tables 1 and 2.
Means and SDs on the NEPSY test for children with bipolar affective disorder
Means and SDs for the Woodcock-Johnson Psychoeducational Battery in children with bipolar affective disorder
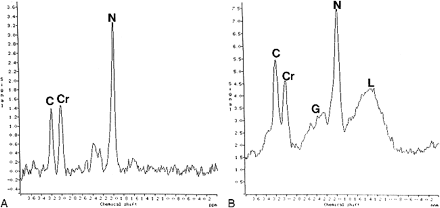
NAA/Cr in the frontal lobes of BPAD subjects (n = 10) was not significantly different (left to right) from that of control subjects (n = 10) (Tables 3 and 4). Likewise, Cho/Cr in the frontal lobes of BPAD subjects showed no significant difference (left to right) from that in control subjects. Glu/Gln with respect to Cr was significantly higher in the frontal lobes of children with BPAD than in control subjects (Fig 2). Lipids were not seen in the control group but were identified in both frontal lobes in seven of the 10 children with BPAD. Lipid levels were higher in the left than in the right frontal lobe (Fig 2B).
Metabolite ratios in the frontal lobes of children with bipolar affective disorder (BPAD) (n = 10) and unaffected control subjects (n = 10)
Metabolite ratios in the basal ganglia for children with bipolar affective disorder (BPAD) (n = 10) and unaffected control subjects (n = 10)
MR spectra from the frontal lobes.
A, Proton spectra from the left frontal lobe in a control subject show normal metabolites. C, choline; Cr, creatine; N, NAA.
B, Proton spectra from the left frontal lobe in a patient with BPAD show prominent resonances between 2.1 and 2.5 ppm, corresponding to Glu/Gln (G). The resonances between 0.8 and 1.8 ppm correspond to lipids (L). C, choline; Cr, creatine; N, NAA.
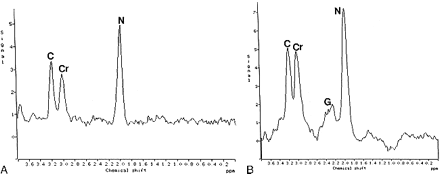
NAA/Cr in the temporal lobes of BPAD subjects (n = 9) showed no significant difference (left to right) from that in control subjects (n = 10) (Tables 3 and 4). Cho/Cr in the basal ganglia of the children with BPAD was not significantly different (left to right) from that in control subjects. Glu/Gln with respect to Cr was significantly elevated in BPAD children as compared with the control group (Fig 3). Lipids in these regions were not present in either group of children. In five of the BPAD children in whom 2D MR spectroscopic studies were obtained (with small volumes of interest encompassing gray and/or white matter), lipid levels were elevated in both frontal lobes, predominantly on the left (Fig 4).
MR spectra from the temporal lobes.
A, Proton spectra from the left temporal lobe in a control subject show normal metabolites.
B, Proton spectra from the left temporal lobe in a patient with BPAD show prominent Glu/Gln (G) but no lipids. C, choline; Cr, creatine; N, NAA.
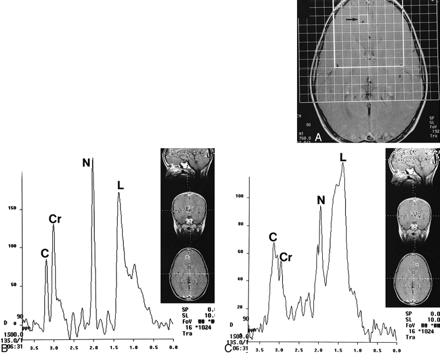
MR spectra from the frontal lobes in a patient with BPAD obtained with smaller voxels.
A, Axial T1-weighted localizer image with grid shows dense. White square outlines the area of analysis. Study was obtained with 2D PRESS technique (TE = 135). Note location of individual voxel (arrow), corresponding to spectra in B.
B, Proton spectra from voxel (arrow, A; 1 × 1 × 1.5 cm) show marked elevation of lipids (L). Note that voxel is far away from any fat-containing structure, precluding extraneous contamination. C, choline; Cr, creatine; N, NAA.
C, Proton spectra of individual voxel placed contralateral and in nearly a mirror position to B still show marked elevation of lipids (L). Again, voxel is placed away from fat-containing structures that could conceivably contaminate it. C, choline; Cr, creatine; N, NAA.
Discussion
Phosphorus-31 MR spectroscopy in adults with BPAD has shown that the ratio of phosphomonoesters (PME) to total peak is elevated during the manic state as compared with the euthymic state (5). The PME-to-phosphodiesters ratio is also higher in the manic state than in the euthymic state and in relation to unaffected control subjects. In bipolar patients, PME is higher in the frontal lobes during the depressed state than during the euthymic state. Patients with BPAD have lower PME in the euthymic state relative to unaffected control subjects and in relation to subjects experiencing major depression. Phosphorus-31 and lithium-7 MR spectroscopy have been used to compare patients in the manic and euthymic states and to compare BPAD patients with age- and sex-matched unaffected control subjects (3). PME has been found to be significantly increased in the manic state relative to the euthymic state and is lower in BPAD patients in the euthymic state than in control subjects. Lithium treatment does not affect the level of PME.
Proton MR spectroscopy has been used to study the basal ganglia in 40 patients with affective disorder (4). During the depressive and euthymic states, these patients had significantly higher levels of Cho/Cr and Cho/NAA than did unaffected control subjects. Other investigators have shown increased NAA/Cr, Cho/Cr, and inositol/Cr in bipolar patients taking lithium (6). Abnormalities in Cho metabolism in rapid-cycling BPAD adults have also been reported (6). In a small number of severely depressed patients before and after electroconvulsive therapy (ECT), an increase in lipid levels in the medial frontal regions was noted as early as90 minutes after ECT (9). The level of lipids decreased 32 hours later.
These data obtained in adults raise the possibility of using MR spectroscopy to detect alterations in brain metabolites in children with BPAD. On the basis of published observations, we selected regions of interest in the right and left frontal and temporal lobes (3–5). Neuropsychological testing was used to establish the functioning capability of these areas. Studies in adults with BPAD have used neuropsychological testing and differences in response to ECT to try to localize abnormalities (10). A review of 25 published papers indicates that the right hemisphere (generally the nondominant one) is preferentially impaired in BPAD, with cerebral disorganization greater during mania than during depression (11). Eighteen of the 25 studies showed dysfunction in the right cerebral hemisphere.
Neuroimaging techniques have been used to show structural and neurometabolic alterations in adults with BPAD. These alterations include cortical atrophy, sulcal widening, cerebellar vermis atrophy, and an enlarged third ventricle (10). T2-weighted MR imaging has shown nonspecific cortical atrophy in the periventricular white matter hyperintensities. A survey of the literature on neuromorphometry of affective disorders revealed that, in adults with BPAD, there is enlargement of the lateral and third ventricles, decreased cerebellar size, deep white matter T2 hyperintensities, and temporal lobe asymmetry (10). MR imaging alterations, mostly asymmetry in the size of the frontal lobes, have been demonstrated in a small number of children with BPAD (12). A retrospective review of MR imaging studies in 65 children and adolescents with affective disorders showed similar findings (2). Thus, brain alterations as detected by MR imaging occur in both adults and children.
Our data indicate that MR spectroscopy may hold promise for gaining an understanding of some neurometabolic alterations in children with BPAD. The presence of increased levels of Glu/Gln, which are excitatory amino acids, suggests a possible dysregulation in neurotransmitter activity in the frontal and temporal lobes. In a recent review, the significance of increased Glu on the developing brain and its relation to the severity and chronicity of BPAD in children were explored (13). Constant or even intermittent exposure to abnormal levels of excitatory amino acids may explain the difficulty in regulating BPAD pharmacologically. A technique for editing Glu/Gln on MR spectroscopic studies would have been desirable but was not available to us at the time of this study.
We found that 70% of our BPAD patients had large lipid resonances in both frontal regions but not in the temporal lobes. In studies using our technique (TE = 135), lipids are not normally present unless the sampled volume is near fat-containing tissues, such as the scalp and orbits (14). In our studies of five patients in whom we used smaller voxels, we found the presence of elevated lipids in areas far removed from those that could conceivably have been contaminated by extraneous lipid resonances arising from the scalp. Thus, we believe that the presence of lipids is not due to contamination by tissues outside the sampled voxel; rather, the elevation of cerebral lipids may be due to either increased degradation of neuronal membranes or to changes in the fluidity of the lipid components of the neuronal membrane, which increases their isotropic motion and allows visualization of their components at a long TE, such as the one used here. Elevation of brain lipids as seen by MR spectroscopy has been documented in the frontal lobes of autistic children and in patients with electrically induced or spontaneous seizures (15, 16). In a recent study, patients with schizophrenia were found to have slightly elevated Cho/Cr ratios in the frontal lobes as compared with the temporal lobes (17), and NAA/Cr was lower in the frontal and temporal lobes of the schizophrenic patients than in those of control subjects. In that study, lipids were also seen, particularly in the frontal regions. The authors used short TEs, so the small amount of lipids seen in their spectra was most likely due to extraneous fat contamination.
Conclusion
MR spectroscopy revealed metabolic alterations in a small group of children with BPAD. These changes were characterized by the presence of increased Glu/Gln levels in the sampled areas of the frontal and temporal lobes and by increased lipids in the frontal lobes as compared with a group of control subjects. The nature of these changes is not clear, and it remains to be proved whether they are typical of BPAD and whether they can be used as an aid in clinical diagnosis or as a screening technique.
Footnotes
↵1 Address reprint requests to Mauricio Castillo, MD, Department of Radiology, University of North Carolina School of Medicine, Campus Box 7510, Chapel Hill, NC 27599.
References
- Received August 16, 1999.
- Accepted after revision January 12, 2000.
- Copyright © American Society of Neuroradiology