Abstract
BACKGROUND AND PURPOSE: In patients with suspected subarachnoid hemorrhage (SAH) and negative CT findings, the iatrogenic introduction of RBCs into the CSF during lumbar puncture may lead to a misdiagnosis. We tested the hypothesis that the risk of traumatic lumbar puncture is lower with the fluoroscopy-guided technique than with the standard bedside technique.
METHODS: Data were collected retrospectively from two populations: adult inpatients undergoing standard bedside lumbar puncture for any reason and adult patients undergoing fluoroscopy-guided lumbar puncture for myelography. Patients with SAH and CSF samples with significant abnormalities other than erythrocytosis (ie, CSF leukocytosis, xanthochromia, or elevated protein) were excluded. In all, 1489 bedside procedures and 723 fluoroscopy-guided procedures met the criteria.
RESULTS: We found a significant difference in the level of iatrogenic CSF erythrocytosis produced by the two procedures. Using a cutoff of 1000 cells/mm3, the frequency of traumatic lumbar puncture was 10.1% for bedside lumbar puncture and 3.5% for fluoroscopy-guided lumbar puncture. With fluoroscopic guidance, the frequency of a traumatic tap varied significantly with the operator, ranging from 0% to 24%.
CONCLUSION: The use of fluoroscopy-guided lumbar puncture in patients with suspected SAH and negative CT findings should reduce the frequency of false-positive diagnoses of acute SAH as well as the number of unnecessary angiograms for patients with suspected SAH but no underlying intracranial vascular malformation.
Lumbar puncture is the diagnostic procedure of choice in patients with suspected subarachnoid hemorrhage (SAH) but with no evidence of blood on CT scans of the head. Lumbar puncture has been supplanted by CT as the primary diagnostic technique in patients with suspected SAH, because lumbar puncture provides no information about the degree and location of SAH or the sequela of hemorrhage in the cerebral parenchyma. However, CSF sampling by lumbar puncture is more sensitive than CT for SAH (1, 2). Unfortunately, traumatic lumbar puncture (ie, CSF erythrocytosis arising solely from the procedure) leads to false-positive diagnoses. Because SAH often reflects the presence of aneurysms or other cerebral vascular malformations, patients with a positive lumbar puncture usually undergo one or even two diagnostic cerebral angiograms. Cerebral angiography is a costly procedure with significant potential morbidity. Therefore, any means of decreasing the rate of traumatic lumbar puncture would decrease the morbidity, discomfort, and cost associated with the evaluation of suspected SAH.
Fluoroscopic guidance has the potential to decrease the frequency of traumatic lumbar puncture. While fluoroscopy is rarely needed to access the subarachnoid space with a spinal needle, it is routinely used for difficult cases in which bedside lumbar puncture has failed. Fluoroscopy shows the bony structures of the lumbar spine and provides real-time information about the position of the needle as it is being inserted. With fluoroscopy it is almost always possible to access the subarachnoid space with a single pass of the spinal needle, minimizing trauma to paraspinal and epidural tissues.
We tested the hypothesis that fluoroscopy-guided lumbar puncture yields a decreased rate of traumatic tap by comparing the levels of CSF erythrocytosis in patients undergoing a fluoroscopy-guided procedure with those undergoing a standard bedside procedure.
Methods
Data were collected retrospectively for a 3-year period at our hospital from two populations: adult inpatients undergoing standard bedside lumbar puncture for any reason and adult patients undergoing fluoroscopic lumbar puncture for myelography. The former population was identified using a clinical patient information database, the latter from the radiology scheduling database. Patients were included only if there was no clinical diagnosis of SAH and only if there was no other significant CSF abnormality; that is, no CSF leukocytosis greater than 20 cells/mm3, no elevation of CSF protein greater than 75 mg/dL, and no xanthochromia. The CSF protein levels and leukocyte counts used to make this determination were corrected for the erythrocyte count in the sample using standard formulas (3). When multiple tubes were drawn, erythrocyte levels were determined from the first tube. Evaluation of serial sampling was not possible because only one cell count was performed for the fluoroscopy-guided cases. By protocol, the first tube was always used for this purpose. Furthermore, since none of the bedside lumbar punctures was performed for suspected SAH, serial sampling was not consistently performed. Xanthochromia was determined by visual inspection.
A third group, which included patients with negative CT findings and a diagnosis of SAH based on the results of a lumbar puncture, was identified from our hospital's radiology records of patients who had undergone cerebral angiography over the past 3 years. Collection of the patient data was approved by our hospital's committee on research, subcommittee on human studies.
Statistical analysis of the difference between fluoroscopy-guided and bedside lumbar punctures was performed in three ways. First, because the data deviated markedly from a normal distribution, log transformation of the RBCs (y′ = log[y+1]) was used to bring the data closer to a normal distribution. An unpaired Student's t test was applied to the data. Second, for the same reason, the untransformed data were analyzed using the nonparametric Mann-Whitney U test. Third, to test the differences between the groups most practically, cases were stratified into one of two groups, those above and those below an arbitrarily chosen RBC count of either 400 or 1000. A sample with a count above the specified level was considered a traumatic lumbar puncture. Differences between the two groups were then analyzed with the χ2 test. Statistical analysis of the differences between operators was limited to a subset of the fluoroscopy-guided group. Operators who had performed more than 10 lumbar punctures were included. Differences were assessed using two-way analysis of variance on log-transformed data and the nonparametric Kruskal-Wallis test on untransformed data. Information on operator identity and experience was only available for the fluoroscopy-guided procedures.
A preliminary evaluation of the cost of this procedure was performed from data on patient charges for standard lumbar puncture, fluoroscopy-guided lumbar puncture, and cerebral angiography at our institution. Both hospital and physician charges were included.
Results
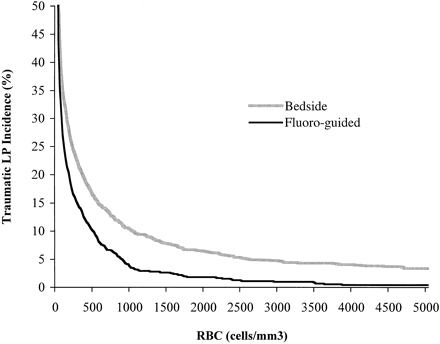
In all, 1489 bedside lumbar punctures and 723 fluoroscopy-guided lumbar punctures met the above criteria. Samples from fluoroscopy-guided procedures consistently showed lower levels of erythrocytes than those from bedside procedures. There was a significant difference between the two groups using either Student's t test of the log-transformed data (P = .000002) or the Mann-Whitney U test of untransformed data (P = .0001). Although there was no absolute RBC count that defined a traumatic lumbar puncture, Table 1 shows the results when levels of either 400 or 1000 were used to classify patients into traumatic and nontraumatic groups. The use of fluoroscopy-guided lumbar puncture resulted in a 37% or 65% decrease in the frequency of traumatic tap when applying the criteria of 400 and 1000 cells/mm3, respectively. This difference was statistically significant, with P < .00005 for either criterion. The frequency of traumatic lumbar puncture is plotted in Figure 1 as a function of the erythrocyte count selected to distinguish a normal from a traumatic procedure. As one varies the level used to define traumatic lumbar puncture from 100 to 2000, the relative decrease in traumatic lumbar puncture that can be achieved with fluoroscopic guidance varies from 26% to 72%.
Frequency of traumatic lumbar puncture for bedside and fluoroscopy-guided methods
Cumulative percentage of CSF samples with a CSF erythrocyte count above an arbitrarily chosen level. The x-axis represents the arbitrarily chosen erythrocyte count above which a lumbar puncture (LP) is considered traumatic. The y-axis represents the percentage of lumbar punctures that had an erythrocyte count above this level
The fluoroscopy-guided lumbar punctures were performed by 37 physicians. These operators included radiology residents, neuroradiology fellows, neuroradiology staff, and neurology staff. Most of the lumbar punctures were performed by neuroradiology fellows under the supervision of staff. Operators with an experience of more than 10 procedures showed differences in levels of CSF erythrocytosis ranging from 0% to 25%. We found a significant operator effect on CSF RBC counts using either a two-way analysis of variance on log-transformed data (P = .005) or a Kruskal-Wallis test (P = .004). The level of training (stratified as radiology resident, first-year neuroradiology fellow, second-year neuroradiology fellow, and attending) had no significant effect on the levels of CSF erythrocytosis.
The patient charges (in U.S. dollars) for standard lumbar puncture, fluoroscopy-guided lumbar puncture, and three-vessel cerebral angiography were $393, $559, and $5367, respectively.
Table 2 shows the results of CSF analysis in the 13 patients admitted to our hospital with negative CT findings and positive lumbar puncture. All these patients underwent cerebral angiography, which identified a single anterior communicating artery aneurysm in one patient and multiple small aneurysms about the circle of Willis in a second patient.
Results of CSF analysis in patients with negative CT findings and positive lumbar puncture
Discussion
This project arose from two observations from our clinical practice. First, that a small but significant number of diagnostic cerebral angiograms were needed for patients with a history suggestive of SAH in whom a head CT scan was negative yet lumbar puncture showed elevated RBC counts. We performed 13 such angiograms over the past 3 years, only two of which showed a vascular malformation, a rate greater than that expected from the frequency of asymptomatic aneurysms in the general population but much less than that for patients with SAH demonstrated on CT studies (4). Second, that the rate of traumatic lumbar puncture seemed to be very low for some operators using fluoroscopic guidance (an observation that was confirmed by the results of this study).
Fluoroscopy-guided lumbar puncture enjoys important advantages that make it easier to avoid trauma. The introduction of blood into a CSF sample during lumbar puncture may occur at many points. The needle must pass through cutaneous, subcutaneous, muscular, fascial, epidural, and dural tissues to reach the subarachnoid space. Veins are present throughout this path, along the periosteum, in the thecal sac, and in the anterior epidural space. The largest vascular structures are in the epidural space and the largest of these are anterior to the thecal sac, immediately behind the vertebral body. Minimizing disruption of vascular structures is best accomplished by accessing the subarachnoid space with a single pass of the needle and by avoiding entirely the anterior epidural venous plexus. Fluoroscopy makes it much easier to do so. Bedside lumbar puncture relies on manual and mental estimation of the bony anatomy to guide the needle in the lateral and craniocaudal directions as well as on frequent stylet removal or feel of the needle as it pierces the dura to guide the needle in the anteroposterior direction. With biplane fluoroscopy, it is possible to precisely position the needle in 3D space at all times. No mental estimation or subtle tactile sense is needed. Bony structures can be avoided and, with care, it is always possible to avoid entirely the anterior epidural space.
This study provides the most complete quantitative assessment to date of the frequency of traumatic lumbar puncture, which is often cited as 20% (5). However, the article from which this figure appears to have originated makes no mention of how this number was determined. The determination of traumatic tap is often made at the bedside by the presence of a pink or red tint to the sample. The level of erythrocytes necessary to produce such coloration varies from 700 to 6000 cells/mm3 (6). Using the lower of these limits and the data from our study, the frequency of visibly traumatic lumbar puncture in our study would be less than 6.6% for fluoroscopy-guided procedures and 14% for bedside procedures.
The desire to distinguish among negative, traumatic, and positive lumbar punctures raises an important question; that is, what is the erythrocyte level beyond which one must consider either traumatic lumbar puncture or SAH? In texts and papers on this subject, the level of erythrocytes above which a lumbar puncture is considered positive is chosen arbitrarily. In many cases a pink or yellow tinge on visual inspection is used. When quantitative levels are specified, the level of erythrocytes varies from 400 to 1000 cells/mm3 (7–10). Although, in theory, even a single RBC in a CSF sample could represent SAH, in practice most lumbar punctures in known SAH have high levels of erythrocytes. A hemorrhage as small as 0.3 mL will result in erythrocyte levels as high as 10,000 cells/mm3 (11). In the era before CT, Merritt and Fremont-Smith (3) reported that erythrocyte concentrations in 47 patients with SAH varied from 1000 to 3.5 million cells/mm3. In the CT-negative cases presenting to our hospital, the level of erythrocytes in the angiography-positive or xanthochromia-positive lumbar puncture-diagnosed cases was always greater than 8000 cells/mm3 (Table 2). In traumatic lumbar puncture, the levels of erythrocytes are generally much lower, as demonstrated in the current study. Thus, it is possible to select an erythrocyte level that will exclude most cases of traumatic lumbar puncture. A level of 1000 cells/mm3 will include almost all true SAH and limit the false-positive rate to less than 5% when fluoroscopic guidance is used.
The difficulty in using erythrocyte levels to distinguish traumatic lumbar puncture from SAH has led to the use of other indexes of SAH. These include traditional methods, such as erythrocyte counts in serial CSF samples and xanthochromia, as well as more recently developed assays, such as D-dimer and ferritin. Unfortunately, each of these methods has limitations and these limitations reinforce the importance of a nontraumatic lumbar puncture technique.
Traditional methods of distinguishing traumatic lumbar puncture from SAH are not satisfactory in many cases. Serial sampling of CSF has been used and is still cited in many neurologic and neurosurgical texts. The clearing of erythrocytes in the later samples, as determined by decreasing RBC counts, has been thought to suggest traumatic lumbar puncture. While this phenomenon is true on average, in any individual case it is not sufficiently reliable to exclude SAH (10, 12). Xanthochromia has been used as the chief means of distinguishing traumatic lumbar puncture from SAH. Xanthochromia is a reflection of the presence of erythrocyte breakdown products in the CSF, chiefly oxyhemoglobin and bilirubin. Since it takes time for these products to be released or to form, they are present only in small amounts in the bloody CSF of a traumatic lumbar puncture (13). Thus, when xanthochromia is present, hemorrhage has probably occurred before the current lumbar puncture has been performed. Unfortunately, it takes about 12 hours from the onset of SAH for xanthochromia to approach 100% sensitivity (14). Furthermore, there is controversy concerning the method by which xanthochromia should be determined. The studies demonstrating a high sensitivity of xanthochromia for SAH used a spectrophotometric assay (14). Many hospitals, however, rely on visual inspection of CSF supernatant to determine xanthochromia, (15) and this method is probably not sufficiently sensitive to detect xanthochromia in many cases of SAH (1). On the other hand, the high sensitivity of the spectrophotometric method may lead to false-positive studies, again leading to unnecessary angiograms (9, 16, 17).
Because of the 12-hour delay needed to ensure high sensitivity for xanthochromic determination of SAH, Vermeulen and van Gijn (1) have advocated that lumbar puncture be deferred until 12 hours after the onset of symptoms. However, this approach has significant potential costs for patient well-being. The waiting period would delay definitive diagnosis and treatment in patients with intracerebral aneurysms. Since the possibility of a rebleed, potentially a catastrophic one, is greatest in the first 24 hours after the initial hemorrhage (18), such a delay is inappropriate. The proposed delay would also prolong the patient's stay in the emergency department or hospital, at significant monetary cost.
More recently, other laboratory methods to distinguish traumatic lumbar puncture from SAH have been proposed. The presence of ferritin and D-dimer assay in CSF is more common in SAH than in traumatic lumbar puncture (10, 19). The earliest studies suggested very high sensitivity and specificity, but more recent series (9, 16) have reported a significant proportion of false-positive and false-negative results. The authors of the more recent series have suggested that these lab studies do not provide useful additional information beyond the determination of xanthochromia. All these studies and those that used xanthochromia as a means of distinguishing traumatic lumbar puncture from SAH relied on a small number of cases and were complicated by the time dependence of substance concentration in CSF and by the absence of an independent reference standard for SAH.
Imaging methods may someday provide another means of avoiding unnecessary cerebral angiography. CT angiography, a technique growing in popularity and made possible by helical CT scanners with narrowly collimated beams, is very sensitive for the detection of aneurysms larger than 3 mm (20, 21). While the presence of SAH cannot be excluded by CT angiography, patients with suspected SAH but negative CT findings for hemorrhage and negative CT angiographic results for aneurysm may be at sufficiently low risk for a rebleeding cerebral aneurysm that no further investigation or therapy is warranted. This hypothesis is untested to date. MR imaging may also provide a means of determining which patients require cerebral angiography. Conventional MR imaging may show a nonaneurysmal cause of SAH (eg, pituitary hemorrhage, spinal dural arteriovenous fistula) (22). One MR technique, the fluid-attenuated inversion-recovery sequence, is sensitive to the presence of SAH, though it lacks specificity and its utility in CT-negative SAH has not been studied directly (23). MR angiography has a sensitivity for cerebral aneurysms that approaches that of CT angiography (24).
The data in this study also suggest that there is potential to reduce even further the frequency of traumatic lumbar puncture. Our findings show that, at least for fluoroscopy-guided lumbar puncture, the choice of operator can have a large effect on the frequency of a traumatic tap. This statistically significant difference between operators suggests that technique and skill play an important role. Experience alone does not seem to be the key factor. By studying the different techniques used by different operators it may be possible to identify the methods most likely to avoid a traumatic lumbar puncture. Here again a fluoroscopy-guided procedure should have an advantage over a bedside procedure, as real-time visualization makes it easier to identify and apply the more successful techniques. We are in the process of evaluating the effect of technique differences on the frequency of traumatic lumbar puncture.
The data obtained to date suggest the following approach in patients with suspected SAH and negative head CT findings: 1) lumbar puncture should be performed by qualified personnel under fluoroscopic guidance; 2) samples should always be tested for xanthochromia (given the low likelihood of traumatic lumbar puncture, spectrophotometry is preferred over visual inspection because of its greater sensitivity); 3) patients presenting sooner than 12 hours after symptom onset should undergo angiography if either CSF xanthochromia or CSF erythrocytosis with more than 1000 cells/mm3 are present; and 4) patients presenting later than 12 hours after symptom onset should undergo angiography only if CSF xanthochromia is present.
Clinical judgment remains an important part of this process, since the likelihood of SAH and underlying vascular malformation is a complex and unknown function of symptoms, signs, time since onset, imaging results, and lumbar puncture results. In difficult cases, CT angiography may provide useful data to tip the scales.
The use of fluoroscopy-guided lumbar puncture could decrease total patient charges. If only the cost of the lumbar puncture is included, the fluoroscopy-guided procedure results in greater patient charges: $160 for each lumbar puncture performed. However, when one considers the decreased number of angiograms, a net savings is realized. Our data suggest that fluoroscopy-guided lumbar puncture would lead to 6.5 fewer angiograms (using a cutoff of 1000 cells/mm3) for each 100 patients evaluated. For these 100 patients, the net patient savings is $18,286. Of course, different results may be obtained if one uses hospital cost or hospital reimbursement as the basis for such calculations.
Conclusion
Fluoroscopic guidance markedly reduces the frequency of traumatic lumbar puncture. Because both traditional and recently proposed methods for distinguishing a traumatic lumbar puncture from SAH are not perfect, the use of fluoroscopy guidance should therefore decrease the number of false-positive diagnoses of SAH. In turn, this should reduce the number of angiograms performed in patients with what is nothing more than a sudden onset or exacerbation of headache. Because of our findings, the practice of the vascular neurosurgery service at our institution has changed: any necessary lumbar punctures obtained for the evaluation of SAH are now requested from the neuroradiology service and performed using fluoroscopic guidance.
Footnotes
References
- Received May 19, 2000.
- Copyright © American Society of Neuroradiology