Abstract
Summary: Calcifying epithelial odontogenic tumors and calcifying odontogenic cysts are rare, benign odontogenic tumors. We report two cases of an exceptional combination of these tumors with either an ameloblastic fibroodontoma or an odontoma.
Calcifying epithelial odontogenic tumors (CEOTs) and calcifying odontogenic cysts (COCs) are rare, benign odontogenic tumors. We report two cases of an exceptional combination of these tumors with either an ameloblastic fibroodontoma or an odontoma. Such a combination, which, to our knowledge, has not previously been reported, led us to review in detail the clinical, radiologic, and microscopic patterns of these tumors and to compare those findings with the present complex classification of CEOT and COC.
Case Reports
Case 1
A 10-year-old boy was referred for assessment of a large asymptomatic mandibular lesion that was discovered on a routine radiographic examination performed to evaluate an unerupted right first molar. Plain radiographs showed a large well-delineated expansile lesion of the mandibular angle with scattered irregular radiopacities with multiple pseudosepta (Fig 1A and B).On CT scans (Fig 1C and D), the lesion showed a combined pattern of a well-circumscribed unilocular radiolucency containing scattered flecks of calcification. Pseudosepta were seen with a large, smooth expansion of the thinned cortical plate, which appeared to be broken on the upper edge of the tumor.
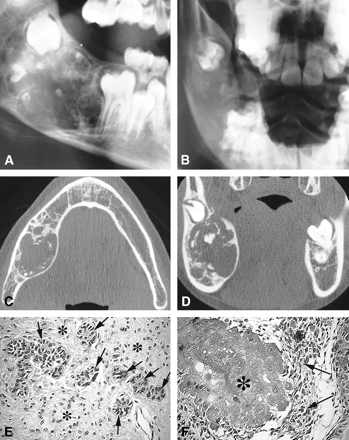
Case 1.
A and B, Plain radiographs. Lateral plain radiograph (A) shows a large expansile mixed radiolucent/radiopaque lesion involving the right mandibular angle. Posteroanterior view of the right mandible (B) shows an anterior displacement of the first molar and a posterior displacement of the germ of the second molar. Scattered calcifications are seen.
C and D, CT scans. Axial view of the mandible (C) shows a large expansile radiolucent lesion of the mandibular angle containing scattered calcifications. Coronal view (D) shows a localized rupture of the thinned cortical plate with a posterosuperior displacement of the germ of the second molar.
E and F, Microscopic sections. These photomicrographs show the association of an ameloblastic fibroodontoma (E) with a CEOT (F) (hematoxylin-eosin, original magnification ×200). The ameloblastic fibroodontoma (E) has islands of epithelial cells that exhibit an enamel organlike arrangement (arrows) seen infiltrating the connective component of the tumor, similar to the dental pulp (asterisk). The CEOT (Pindborg tumor) (F) has epithelial cells (arrows) seen with foci of amyloidosis (asterisk)
On surgical exploration, the lesion, which was located in the superior break of the cortical plate, appeared as a large, irregular, whitish mass with numerous hard nodules. The lesion was completely enucleated.
Macroscopic examination revealed two fragments (2.8 × 1.7 cm and 3.5 × 2.5 cm, respectively) with variable consistency, sometimes soft, sometimes hard, with calcificlike areas. The microscopic sections (Fig 1E and F) showed two more or less intricate patterns: first, there were large areas of myxoid connective tissue containing spindle- or star-shaped cells. Found within these areas were ameloblastic crests or follicles with localized production of enamel, dentine organized in small teeth with a central pulp. Second, islands of epithelial cells were embedded within the latticework, sometimes with a clarified or ghostlike appearance and numerous calcifications. Some amount of an amorphous amyloidlike material was also present, which showed an apple green birefringence and positivity via the alkaline Congo red procedure, while under a fluorescent microscope it also revealed positive thioflavin T staining for amyloid.
The diagnosis was combined ameloblastic fibroodontoma and CEOT. The patient is currently free of any recurrence 3 years after surgery, and follow-up plain radiography showed nearly complete healing of the lesion.
Case 2
A 14-year-old girl presented with a lingual swelling on the left side of the mandible. Physical examination revealed a well-defined swelling, extending from the movable first molar to the angle, with slight pain at provocation. A plain radiograph (Fig 2A) revealed a large well-defined expansile unilocular radiolucency, containing several small peripheral radiopacities. Focal resorption of the first molar was noted with an inferior displacement of the unerupted second molar, while the germ of the third molar was ectopically placed in the upper ramus. CT specified the pattern of this 5 × 2.2-cm well-delineated expansile lesion with a large smooth expansion of the thinned cortical plates seen predominately on the lingual edge; small peripheral calcifications were also present (Fig 2B). At MR imaging, the lesion showed a homogeneously and slightly hyperintense signal on noncontrast T1-weighted sequences (Fig 2C) and a markedly hyperintense signal on T2-weighted sequences after intravenous injection of contrast material (Fig 2D).
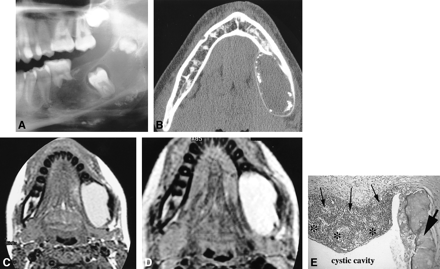
Case 2.
A, Lateral plain radiograph of the mandible shows a large well-defined expansile radiolucency containing several small peripheral radiopacities. Focal resorption of the first molar with inferior displacement of the unerupted second molar is seen. Note the upper displacement of the germ of the third molar.
B, Axial CT view of the mandible shows a well-delineated expansile lesion with a large, smooth expansion of the thinned cortical plates. Small peripheral calcifications are seen.
C and D, MR images. Axial noncontrast spin-echo T1-weighted image (420/11/2) (C) shows a homogeneous, slightly hyperintense lesion. Axial fast spin-echo T2-weighted image (4000/104/1) (D) shows a markedly hyperintense lesion.
E, Photomicrograph shows the characteristic patterns of a COC close to a small odontoma (hematoxylin-eosin, original magnification ×200). Epithelial cyst lining (thin arrows) is seen with focal areas of necrosis (asterisks). Dentinoid component of the odontoma (thick arrow) is associated
On surgical exploration, the lesion appeared as a well-defined cyst containing brownish liquid with a nonadhering membrane covered with granulations thought to correspond to the radiographic calcifications. The lesion was completely enucleated. The surgical specimen was a cystic shell with several foci of calcifications. Pathologic examination (Fig 2E) revealed a stratified epithelial lining with an ameloblasticlike basal layer. Several groups of ghost cells were distinguished inside this lining and they were sometimes mineralized. Dentinoid was also noticed in contact with the basal layer of the epithelium. Close to this lining, a small odontoma with dental pulp, enamel, and dentine could also be seen.
The diagnosis was combined COC and odontoma. The patient is currently free of any recurrence 3 years after surgery.
Discussion
Odontogenesis is a complex interplay between epithelial elements and those that are derived from ectomesenchyme. Tooth formation is initially evidenced by proliferation of the dental lamina, an epithelial structure found in the primitive oral mucosal epithelium. This dental lamina can proliferate only in the presence of the underlying ectomesenchyme, which thus has an inductive effect on the dental lamina forming the enamel organ. The enamel organ is a three-dimensional structure that has four components: the inner enamel epithelium, the stratum intermedium, the stellate reticulum, and the outer enamel epithelium. A process called induction begins when the cells of the dental lamina closest to the inner enamel epithelium differentiate into odontoblasts and begin to form dentin. The formation of dentin again reciprocates in the inductive ability to stimulate the preameloblasts of the inner enamel epithelium, differentiating them into ameloblasts, which begin to secrete enamel.
CEOT (also called Pindborg tumor) and COC are rare, benign tumors involving the odontogenic apparatus. Over the last decades, classification and individualization of the benign odontogenic tumors have seeded confusion and controversy in the literature. CEOT was only distinguished from ameloblastoma in 1955 by the Danish pathologist Pindborg (1), while COC was first described in 1962 by Gorlin et al (2). In 1971, a histologic typing of “odontogenic tumours, jaw cysts, and allied lesions” by the World Health Organization (WHO) classified neoplasms and other tumors of the odontogenic apparatus simply as benign or malignant (3). In 1992, a new WHO international classification system divided these benign tumors into three groups on the basis of their microscopic patterns, their embryonal origins, and their interactions with odontogenic tissues (4, 5) (Table). The first group included the epithelial tumors, in which there is odontogenic epithelium without odontogenic ectomesenchyme, such as CEOT (as in case 1); the second group included the mixed odontogenic tumors, in which there is odontogenic epithelium with odontogenic ectomesenchyme with or without dental hard tissue formation, such as ameloblastic fibroodontoma (as in case 1), COC (as in case 2), and complex odontoma (also as in case 2); and the third group included those tumors in which there is odontogenic ectomesenchyme with or without odontogenic epithelium.
Benign neoplasms related to the odontogenic apparatus
The odontogenic tumors thus seem to reproduce in a more or less acquired way the different steps of odontogenesis. The first group of epithelial tumors is thought to be histologically related to remnants of the odontogenic epithelium (dental lamina, enamel organ, root sheath of Hertwig), while the second group of mixed tumors is composed of both epithelial- and mesenchymal-derived tissues (6–8). The critical factor in understanding the mixed odontogenic tumors as a group and their relationship with tooth formation is in recognizing the inductive and reciprocally inductive influences of one tissue on another. The present hypothesis is that the tumors included in the last group recapitulate some stages of odontogenesis, ranging from the earliest phase (ameloblastic fibroma) to that in which there is a high degree of histodifferentiation (odontomas). On the other hand, it is thought that the lesions in the first group of epithelial odontogenic tumors arise from residue of cells issued from actively growing dental lamina that are present within the jaws for a considerable time after birth. In this respect, our two cases, and in particular case 1, are interesting in that they represent an association of tumors histologically classified into two different groups. To our knowledge, no such association has previously been reported in the literature. Although the clinical, radiologic, and microscopic association of a COC with an odontoma has previously been described in detail (9–11).
More rarely, COCs have been reported to be associated with other odontogenic tumors of its group. Such tumors have been considered to be part of the entire lesion and related to the ability of the COC to induce the formation of mature dental tissue. This combination of one tumor, considered to be an intermediate differentiated lesion of its mixed group, with an odontoma, considered to be the most differentiated lesion of this same group and therefore even considered to be a hamartoma, is today regarded as an important argument for evoking the hypothesis of a kind of histomorphologic filiation between all tumors of this group. Conversely, as in our case 1, the association of a CEOT with one of the intermediate mixed-group tumors (ie, ameloblastic fibroodontoma) is quite surprising. In both pathologic and radiologic examinations, these tumors appear within a single intricate lesion, with a homogeneous radiologic appearance, and on microscopic sections as a combination of the two different patterns embedded in the same lesion and not as two different lesions that developed in neighboring areas. This case can thus be related to the only other one described in the literature, in which the authors reported two cases of CEOTs in association with an adenomatoid odontogenic tumor (12). As in our case 1, the intricate tumor patterns (and not a collision of separate tumors in which the cells could not be expected to be so intermingled) suggests that these lesions represent primarily a mixed tumor (adenomatoid odontogenic tumor, or, in our case, an ameloblastic fibroodontoma), in which foci of CEOT had developed. It would not be surprising for this to occur, since these mixed tumors contain stratum intermedium.
In such pathologic processes, it is clearly recognized today that a histologic diagnosis can be quite difficult and that a definitive diagnosis can only be made in conjunction with clinical and radiologic correlation (7). The four histologic specimens obtained in our two cases proved to be benign, slow-growing tumors, the clinical diagnosis of which is most often made in the presence of a (frequently) painless lesion, which may cause swelling or displacement of teeth or that prevents tooth eruption (7, 13). An analysis of demographic features shows that CEOT is considered to be a tumor of adults, with most patients between the ages of 30 and 50 years; however, in ameloblastic fibroodontoma, the mean age is 8 years (6, 7). The patient reported in case 1 was only 10 years old, and it would seem that this case not only represents a microscopic mixture, but also a hybrid clinical presentation. Similarly, the mean age of diagnosis of COCs is 30 years, while for patients with an odontoma-associated type, it is relatively lower (17 years) (9), and, in our case 2, the patient was 14 years old.
Most of the tumors represented in this classification are rare, and reports of such lesions are sparse. So, except for the most frequently occurring tumors, such as ameloblastomas or odontomas, their radiologic patterns have not been well characterized and, in most cases, are described on plain radiographs (6, 7, 9, 12–17). In our two cases, plain radiographs showed a well-delineated expansile lesion with thinned cortical plates, suggesting a benign slow-growing tumor. In case 1, a small break of the superior cortical plate was more apparent on CT scans as a fairly pronounced thinning of the cortical plate rather than as an aggressive rupture of the bone, owing to the progressive thinning of the bone, with an absence of any neighboring mucosal invasion. This focal bony rupture, which was also found on surgical examination, is quite rare in such benign odontogenic tumors, found in less than 7% of reported cases of COC (9). Such a pattern should be kept in mind as a possible feature of benign odontogenic lesions.
Calcifications were present in our two cases, best detected on CT scans rather than on plain radiographs, appearing as more extensive scattered flecks in case 1 (CEOT with ameloblastic fibroodontoma) and as small peripheral calcifications in case 2 (COC with odontoma). Previous reports of CT patterns of such lesions are quite rare; however, CT may be beneficial in depicting such calcifications, whose presence may be characteristic of these lesions, as they may be obscured on plain radiographs by such surrounding structures as tooth or metal restoratives (14). Detection of such calcifications is essential to guide the differential diagnosis among the great variety of expansile radiolucent lesions of the jaws. Thus, CEOT may show considerable radiologic variations: its appearance may range from a diffuse, poorly demarcated, or well-circumscribed unilocular radiolucency to a combined pattern of radiolucency and radiopacity with small intralesional septa producing a multilocular pattern (6, 13). The more classical pattern, in which flecks of calcification are scattered in an area of radiolucency, appears quite superimposable to our case 1.
In the rare reports of CT patterns in ameloblastic fibroodontoma, calcifications have been described as having two distinct patterns: either small calcifying nodules scattered through the lesion or calcifying spicules radiating from the center of the lesion and displaying a wheel-like appearance; with advancement in tumor maturation, these radiating spicules can fuse to form a massive calcification (7, 14). Despite the microscopic presence of the ameloblastic fibroodontoma in case 1, radiologically, it appeared to be more like a CEOT or, at best, as a hybrid of a CEOT and an ameloblastic fibroodontoma. However, calcifications, which can be suggestive of a diagnosis, may be absent in ameloblastic fibroodontoma, instead showing a totally radiolucent lesion; although dental hard tissues, by definition, must be formed. The degree of mineralization rather than the presence of the matrix accounts for the radiodensity of the lesion (7). COC is also known to display a variable radiologic pattern, appearing as a unilocular or, rarely, a multilocular radiolucency with variable amounts of radiopaque material ranging from tiny flecks (as seen in our case 2) to large masses (9, 16). In case 2, the characteristic patterns of odontoma were not found on radiologic examination owing to its small microscopic size. Detection of such calcifications is therefore crucial in the differential diagnosis of these odontogenic tumors.
To our knowledge, the MR appearance of COCs has not previously been reported in the literature. Its relatively homogeneous, slightly hyperintense T1 signal and hyperintense T2 signal without enhancement after contrast injection seems to be well correlated with the macroscopic appearance, which shows a cystic lesion without a solid nodule; and the brownish appearance of the cystic liquid could be related to minimal bleeding beforehand, thus explaining the T1 signal.
In both our cases, then, the presence of calcifications virtually excluded the diagnosis of ameloblastoma (the most frequent epithelial odontogenic tumor of the jaw, representing 1% of all tumors and cysts of the jaw), in which the presence of calcifications is quite exceptional. Moreover, ameloblastoma characteristically displays a large multilocularity with a mixed pattern of solid and cystic components and strong enhancement of the solid portions of the tumor. Diagnosis may be more difficult in small lesions; however, a solid, enhancing component without calcifications is the rule.
The importance in differentiating ameloblastoma from the other odontogenic tumors lies in the different prognosis each tumor carries and therefore the different therapeutic implications (18–20). Similarly, the other epithelial odontogenic tumors, such as the exceptional and occasionally aggressive squamous odontogenic tumor, can be differentiated from our cases by their absence of calcification within a semicircular or triangular-shaped radiolucency between the roots of the teeth, with lysis of the lamina dura (21, 22). The recently individualized clear cell odontogenic tumor, however, appears radiologically indistinguishable from our cases as a purely radiolucent or as a mixed radiolucent/radiopaque uni- or multilocular lesion with either well-demarcated or poorly defined margins. Such findings are, however, not surprising, as the clear cell odontogenic tumor is today considered the neoplastic counterpart of COC (8). In the same manner, regarding mixed odontogenic tumors, the less differentiated ameloblastic fibroma can be excluded by the absence of calcifications. But the radiologic patterns of the exceptional odontoameloblastoma, like those of the equally rare adenomatoid odontogenic tumor, are indistinguishable from those of our two cases, which is also not surprising considering the steps of histodifferentiation they represent among mixed odontogenic tumors. Only the more anterior and maxillary location of the adenomatoid odontogenic tumor could elicit this diagnosis, since most of the other lesions predominate in the posterior part of the mandible. Clinical and microscopic correlations are therefore crucial, because the aggressive odontoameloblastoma shares a high risk of recurrence with ameloblastoma (23).
Nonetheless, other tumors must still be considered in the differential diagnosis of lesions with mixed radiologic patterns, such as the odontogenic fibroma, which is most often located in the more anterior part of the maxilla (24), and the cementifying and ossifying fibromas, the dense calcified components of which usually show a more radiated appearance.
Conclusion
The radiologic, clinical, and microscopic findings in our two cases represent an unusual hybrid presentation that suggests the possibility of histomorphologic differentiation. In such cases, radiologic patterns can guide the diagnosis and perhaps be integrated into the classification of complex, benign odontogenic tumors.
Footnotes
↵1 Address reprint requests to N. Martin-Duverneuil, Department of Neuroradiology, Charcot, GH Pitié-La Salpêtrière, 47, Bd de l'Hôpital, 75013 Paris, France.
References
- Received September 26, 2000.
- Accepted after revision January 3, 2001.
- Copyright © American Society of Neuroradiology