Abstract
Summary: We present an unusual case of a giant cell reparative granuloma arising from the left temporal lobe area of a 38-year-old man and provide clinical and MR findings. Current diagnosis and treatment options are also discussed.
Giant cell reparative granulomas (GCRG) are benign, reparative, metabolic lesions that may exhibit local aggression and result in extensive tissue destruction in advanced cases (1). Although this relatively uncommon entity has been described most frequently involving the mandible and maxilla, it can also be found in the orbit (2), ethmoid sinus (3), and cranial vault (4). We present a case of GCRG of the temporal bone with associated MR findings.
Case Report
A 38-year-old man was admitted to our medical center with a 2-year history of progressive fullness in his left ear, hearing loss, foul-smelling rhinorrhea, and dull, bilateral headaches. He denied any visual disturbances, tinnitus, nausea, or vomiting. The patient had a decreased ability to smell. Cranial nerves II–XII were grossly intact. No motor or sensory defects were demonstrated. The remainder of the neurologic tests and the general examination were normal.
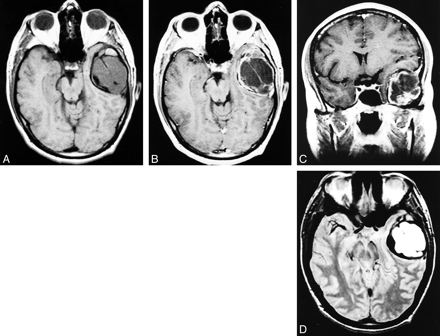
A complete skull series was normal. Contrast-enhanced MR imaging of the brain revealed a 5.4 × 4 × 4-cm extraaxial mass in the left temporal fossa. The mass was isointense on T1-weighted images, hyperintense on T2-weighted images, and appeared well encapsulated, multilobular, and septated and showed capsular enhancement (Fig 1). There was no associated edema, but there was significant mass effect on the adjacent brain parenchyma. On the basis of radiologic findings, the preoperative diagnosis was giant cell tumor.
A, Axial T1-weighted MR image 9400/19/2 [TR/TE/excitations]). An isointense septated extraaxial mass in the left temporal fossa.
B, Axial postcontrast T1-weighted MR image (650/11/2). Well-encapsulated mass with contrast enhancement of the capsule and septations.
C, Coronal postcontrast T1-weighted MR image (650/11/2). Extraaxial mass effect on the adjacent brain parenchyma is seen.
D, Axial T2-weighted MR image (3500/102/2). The mass is hyperintense on the T2-weighted image without associated edema
A left frontotemporal craniotomy yielded a 3 × 3-cm tumor that was beige, tough in consistency, and extradural in location and appeared to originate in the temporal bone. It was firmly attached to the overlying dura and was in one location. It extended through the squamosal portion of the temporal bone, invaded the petrous bone medially, the floor of the temporal fossa inferiorly, and the superior aspect of the mastoid laterally. The lesion was separated from the dura by a blunt and sharp dissection. It was completely resected along with a margin of grossly uninvolved bone.
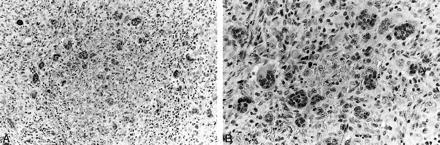
On cut section, the specimen was composed predominantly of firm, beige tissue. Histologically, the tumor specimen consisted of giant cells and similar but mononuclear stromal cells, consistent with a diagnosis of GCRG (Fig 2).
A, Low-power microscopic section of the tumor shows randomly distributed multinucleated giant cells in spindle cell stroma (hematoxylin and eosin staining, magnification ×100).
B, High-power microscopic section with numerous multinucleated giant cells (hematoxylin and eosin staining, magnification ×400)
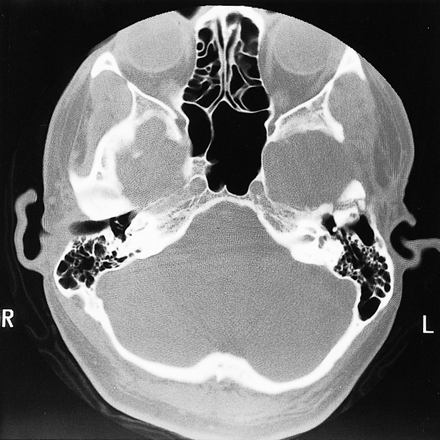
A postoperative CT scan confirmed gross total tumor resection and the patient showed no neurologic deficit at discharge. There was no clinical or CT evidence of tumor recurrence 12 months after surgery (Fig 3).
A 12-month posttherapy CT scan with bone windows (3000/650) shows postoperative changes at the left temporal bone without evidence of tumor recurrence
Discussion
In 1953, Jaffe (5) first described GCRG as benign lesions affecting the mandible and maxilla that were histologically distinct from true giant cell tumors of the bone (1). Originally, the pathogenesis of GCRG was thought to be a hyperplastic reparative reaction to interosseous hemorrhage induced by trauma (6); however, a definite history of trauma has not been verified in cases of GCRG. Other theories on the pathogenesis of GCRG, including infection and developmental causes, have been proposed, but no single theory has gained wide acceptance (6).
Although the most commonly reported radiographic finding is bony destruction with or without a soft tissue mass, GCRG do not have a distinguishing appearance and may mimic other expansile osseous lesions (7–9). Plain film characteristics of cranial GCRG are indistinguishable from other radiolucent skull lesions (10). On CT scans, irregular osseous destruction, calcifications, and contrast enhancement can be seen (8). CT alone, however, is insufficient to differentiate GCRG from other diagnostic possibilities. MR findings of GCRG include low T1 signal, high T2 signal with hypointense septations and variable contrast enhancement (8, 11).
Differential diagnoses of our lesion included giant cell tumors, GCRG, and “brown tumors” of hyperthyroidism. Other less likely osseous lesions with a giant cell component that were considered included aneurysmal bone cysts, nonossifying fibromas, fibrous dysplasias, chondroblastomas, and osteosarcomas (12–14). Giant cell tumors of the bone are hardest to distinguish from GCRG.
Owing to a lack of distinguishing radiologic features of GCRG, biopsy specimens are necessary to make an accurate diagnosis (8). Histologically, they are generally considered benign, although some do exhibit very aggressive behavior (10). These tumors consist of mononuclear, spindle-shaped, stromal cells along with multinucleated giant cells (8, 15). In GCRG, the giant cells tend to cluster and there is abundant new bone formation. In true giant cell tumors, the giant cells are more uniformly distributed, there is no new bone formation, and the mononuclear cells are round or oval. Unfortunately, no current consensus exists regarding the potential for aggressive behavior in a given case of GCRG (14).
The importance of distinguishing GCRG from giant cell tumor lies in the difference in prognosis, with giant cell tumors having a higher incidence of recurrence, metastasis, and malignant transformation (6). Regarding giant cell tumors, cerebral invasion, local recurrence, and late malignant transformation with lung metastatsis have all been reported (9, 10). Reed et al (14) documented a 6% rate of malignant transformation in giant cell tumors of long bones following irradiation and occurring at a mean interval of 13 years. The most common malignant sequelae encountered were fibrosarcoma, osteosarcoma, and malignant fibrous histiocytoma (10). Conversely, GCRG have lower rates of recurrence, ranging from 10% to 15% (6). To date, no documented metastatic transformation has occurred with GCRG (6)
Optimal management of GCRG is complete surgical excision (12, 16). Owing to their location, however, skull base tumors often do not lend themselves to complete excision. Fortunately, long-term remission has been reported with radical resection of accessible tumor when combined with radiotherapy (12, 16).
Regarding the elimination of residual GCRG, the role of adjuvant radiotherapy is controversial. Reports cited by Findlay et al (12) claim that GCRG are not radiosensitive and that radiation is accompanied by a significant risk of sarcomatous transformation. We concur with their review of giant cell reparative granulomas, which concluded that radiation should no be employed until all surgical alternative have been exhausted.
GCRG are uncommon benign lesions most commonly found in the mandible and maxilla. We present an unusual case involving the temporal bone of a 38-year-old man. The MR and pathology findings and treatment options for GCRG are discussed. Although the GCRG does commonly have certain MR characteristics, as discussed herein, the findings are not specific for this tumor and histologic analysis is required for diagnosis.
Footnotes
↵1 Address reprint requests to Francis J. Hahn, MD, Department of Diagnostic Radiology, Division of Neuroradiology, University of Nebraska Medical Center, Omaha, NE 69198-1045.
References
- Received August 14, 2000.
- Copyright © American Society of Neuroradiology