Abstract
BACKGROUND AND PURPOSE: White matter lesions on MR images obtained from patients with acute disseminated encephalomyelitis (ADEM) have been reported to appear shortly after symptom onset, and their resolution has been claimed to parallel recovery. To elucidate the temporal evolution of these lesions and to associate the changes on MR images to the patients' clinical condition, we performed serial MR imaging on patients with ADEM.
METHODS: Several consecutive T2-weighted and fluid-attenuated inversion recovery scans were obtained from four previously healthy adult patients with ADEM within the first days after the onset of symptoms and again during the recovery period. MR imaging was done first on a weekly to biweekly basis and later at 1- to 2-month intervals for up to 8 months.
RESULTS: MR scans of three of these patients did not show any specific abnormalities until several weeks after the onset of the disease. As the lesions later appeared, their number increased during the recovery period.
CONCLUSION: MR imaging performed during the first days after the onset of the disease may not reveal any pathologic findings. The appearance of the ADEM-associated MR imaging changes may be associated with recovery rather than decline. It remains to be studied whether the new MR imaging techniques reveal the lesions associated with ADEM faster than the conventional T2-weighted imaging.
Acute disseminated encephalomyelitis (ADEM) is a severe, acute, demyelinating disease of the CNS. It is usually triggered by an inflammatory response to viral infections and vaccinations. A hemorrhagic, hyperacute variant of ADEM (AHEM/AHLE or Hurst disease) has also been described (1, 2). The course of ADEM is usually monophasic and affects children more commonly than adults (3). The main symptoms are decreased level of consciousness varying from lethargy to coma, convulsions, and multifocal neurologic symptoms such as hemi-, para-, and tetraparesis, cranial nerve palsies, and movement disorders (4–6). In some cases, behavioral changes varying from irritability, depression, delusions, and psychosis may dominate the symptoms (3). CSF is frequently abnormal in the presence of ADEM, with moderately increased leukocyte and protein levels. In AHEM, analysis of CSF usually reveals increased number of erythrocytes. Furthermore, the IgG index is sometimes increased and isoelectric focusing (IEF) of CSF proteins may reveal oligoclonal bands, indicating intrathecal antibody production. The onset of symptoms is usually, but not always, preceded by a prodromal phase associated with fever, myalgia, and malaise. ADEM is associated with a significant mortality of 10%–30%. About 20%–30% of patients who survive are left with neurologic sequelae (6, 7). In MR imaging, ADEM causes multiple sclerosis (MS)-like, but more asymmetrical, white matter lesions (8). In several reports, these lesions have been documented to show up in the first MR imaging scans performed shortly after the first symptoms. Disappearance of these lesions has been suggested to be associated with clinical recovery (1). To better characterize when the ADEM-associated lesions actually appear on MR scans and how the evolution of these lesions is associated with the patients' clinical condition, we performed serial MR imaging on four previously healthy adults with ADEM.
Methods
Patients
The cases presented here are four consecutively admitted adult patients who were treated for ADEM at our institution during the years 1998–1999. All the patients were previously healthy and had no history of drug abuse or alcoholism. Case 1 (38-year-old man) and case 4 (54-year-old woman) were admitted for fever and headache, case 2 (40-year-old man) because of seizures, and case 3 (34-year-old woman), left hemiparesis. Except for case 3, all patients had had a 1- to 2-week prodromal phase of fever, stomach pains, nausea, flu-like symptoms, and headache. All the patients presented here were admitted to the hospital because of a clear or presumed neurologic disease. CSF analysis was done several times for all the patients.
Imaging
Initial CT scans performed at admission failed to show any pathologic findings. Several consecutive MR imaging scans were performed on all four patients. Routine scans included T1- and T2-weighted images (all patients), proton density-weighted (PD) images (patient 1, first scan), and non-contrast fluid-attenuated inversion recovery (FLAIR) images (patients 1, 2, and 4). T2-weighted, PD, and FLAIR images are presented here. The parameters used were 3000/40/1 (TR/TE/TI) for PD imaging, 4000/115/1 for T2-weighted images, and 9002/200/2200/2 (TR/TE/TI/excitations) for FLAIR images. During the acute phase, the MR imaging was performed on a weekly to biweekly basis and later, during the recovery period, at 1- to 4-month intervals. The patients were followed up to 8 months after being admitted to the hospital.
Results
Patients
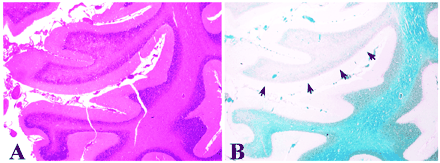
Within a few days after being admitted to the hospital, the patients' condition rapidly deteriorated. During the following weeks, the condition of patients 1, 3, and 4 gradually improved. Patient 2 was treated in the intensive care unit until his death 7 weeks later. Histologic analysis of the autopsy material obtained from patient 4 showed spotty demyelination in the brain parenchyma with perivascular clotting of lymphocytes (not shown). A larger demyelinating lesion was seen in the right cerebellar lobe (Fig 1). The clinical data of the patients presented are summarized in Table 1.
Summary of clinical information
Hematoxylin-eosin (A) and luxol fast blue (B) staining of the lesion seen in the cerebellum of case 2. Hematoxylin-eosin staining shows intact gray matter and some degenerative changes of the white matter. Luxol fast blue staining of myelin (blue) shows a restricted, focal area of demyelination (arrows)
MR Imaging
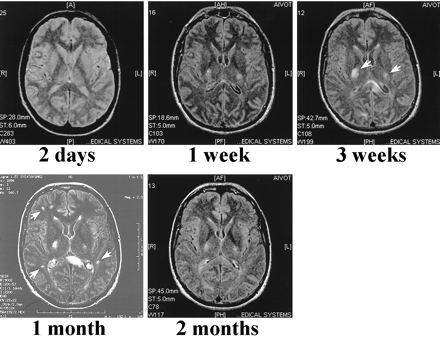
A summary of the MR findings is presented in Table 2. The first two MR scans obtained at 2 days and 1 week from patient 1 did not reveal any major pathologic findings, despite his serious clinical condition (Fig 2). When the patient began to recover, the third scan, done at 3 weeks, revealed a few high-signal areas in the deep white matter. Despite significant recovery, the fourth scan, obtained at 1 month, showed that these lesions had grown in size and number. The new lesions enhanced after contrast medium administration. The last scan, obtained 2 months after admission, showed almost complete resolution of the lesions.
Summary of MR findings at indicated times after admission
PD (3000/40/1 [TR/TE/TI]) (first image) and non-contrast FLAIR (9002/200/2200/2 [TR/TE/TI/excitations]) MR images of case 1 at indicated times after admission to the hospital. The first scan, performed at 2 days, does not reveal any intraparenchymal lesions. In the second scan done 1 week after admission, when the patient was unconscious and connected to a respirator, only two weakly high-signal areas are located in the basal ganglia, which by 3 weeks have grown (arrows). Despite significant recovery, several new lesions are seen in the periventricular white matter 1 month after admission (arrows). By 2 months, almost all the lesions are resolved
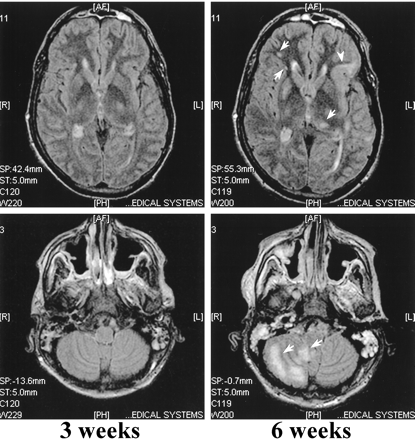
The first MR imaging scans of patient 2, obtained at 2 and 3 weeks, showed a single, minor deficit in the right parietal lobe (not shown). The last scan, obtained at 6 weeks, showed a large subcortical infarct in the left temporal lobe, a few small lesions in the periventricular white matter, and a large subcortical lesion in the right cerebellar hemisphere (Fig 3).
Non-contrast FLAIR (9002/200/2200/2) MR images of case 2 at 3 and 6 weeks after admission. MR imaging performed at 3 weeks does not show any focal lesions, whereas the last MR scan shows a few high-density lesions located in the deep periventricular white matter (arrows) in addition to a subcortical infarct in the left frontoparietal lobe (arrowhead). A large, high-density lesion is located in the right cerebellar hemisphere
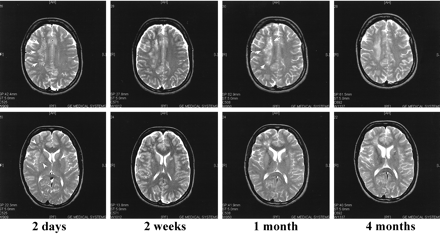
The initial MR imaging scan of patient 3, obtained at 2 days, showed a large asymmetrical white matter lesion (Fig 4). Despite the slight recovery, the size of the lesions had increased on the second scan that was obtained at 2 weeks, and the lesions enhanced after contrast medium administration. On the third scan, evaluated at 1 month, these lesions remained unchanged in size and no longer enhanced after contrast medium administration. Petechial hemorrhage was seen. On the last MR scan, evaluated at 4 months, the lesions had decreased in size. Mild cortical atrophy was noted.
T2-weighted (4000/115/1 [TR/TE/excitation]) MR images of case 3. The first MR scan taken 2 days after admission shows a large lesion in the right centrum semiovale (arrows), as well as in the periventricular white matter and basal ganglia (arrowheads). By 2 weeks, these lesions have grown despite steroid treatment and slight improvement in the patient's condition. By 1 month, some petecchial hemorrhage is seen in the lesions (arrows). On the last MR scan, performed 4 months after admission to the hospital, the lesions are somewhat decreased in size. Note the mild cortical pseudoatrophy likely caused by the steroid treatment
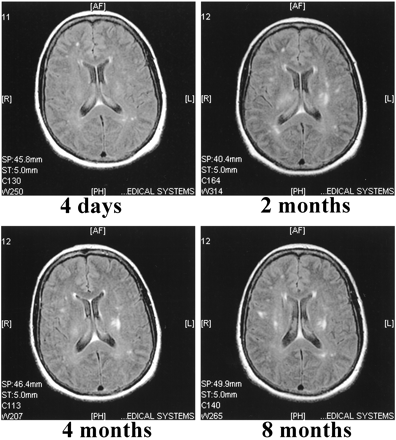
The first scan of patient 4, evaluated at 4 days, showed only a few presumably ischemic lesions in the white matter. Despite her recovery, the second scan, obtained at 2 months, revealed bilateral lesions in the periventricular white matter. The third scan, obtained at 4 months, showed partial resolution of the lesions seen on the previous scan. However, despite her continuing improvement, multiple new lesions had emerged. These lesions remained unchanged on the last scan obtained at 8 months (Fig 5).
Non-contrast FLAIR (9002/200/2200/2) images of case 4. The first MR scan shows a few focal ischemic white matter lesions located in the right frontal and left occipital lobes. The second scan, performed 2 months after admission, reveals several new lesions located in the deep periventricular white matter. These lesions do not show any major resolution in the follow-up scans performed at 4 and 8 months
Discussion
A common feature for all the patients presented here was a rapid deterioration in their condition within a few days after being admitted to the hospital. Most of our patients had had a typical prodromal phase lasting for 1 to 2 weeks. Initial CSF analysis revealed moderately to clearly elevated protein levels and leukocyte counts, which together with acute disorientation, may be interpreted as signs of viral encephalitis. However, the MR imaging findings, together with the characteristic clinical picture, favored the diagnosis of ADEM over viral encephalitis.
The exact etiologic factors that triggered the encephalopathy remained unestablished for all four cases, despite extensive analysis. Patient 1 had had fever and stomach pains for 1 week, and repeated analysis of urine showed constant hematuria, highly suggestive for pyelonephritis. However, repeated analysis of urine for bacterial growth remained negative. Patient 2 had suffered from flu-like symptoms for 1 week, after which he developed pansinusitis. Repeated cultures of the secretions obtained by punctures of paranasal sinuses revealed no specific pathogen. A single sample stained positive for M. tuberculosis, but repeated polymerase chain reaction (PCR) analyses were negative. Furthermore, no bacterial growth in the CSF, with M. tuberculosis included, could be demonstrated by staining, culturing, or PCR. In postmortem analysis, no histologic changes associated with tuberculosis could be seen, but the focal demyelination and perivascular inflammation, together with the MR imaging changes and typical clinical picture, favored the diagnosis of ADEM over tuberculosis. Thus, the single sample stained positive for tuberculosis appeared to be due to contamination. Patient 3 had no prodromal phase, but MR imaging showing petechial hemorrhages, together with erythrocytes in repeated CSF samples, supported the conclusion that this patient had AHEM. Patient 4 was disoriented and had high levels of lymphocytes in CSF, suggestive of viral encephalitis. Demonstration of herpes simplex virus 1 (HSV) by PCR was positive in one CSF sample, but this was not followed by an increase in HSV antibodies in CSF. Thus, the positive result of HSV-PCR appeared to be due to contamination. The IgG-index, together with IEF of CSF proteins, remained pathologic in samples taken 8 months after the onset of the symptoms. Although the CSF findings may take a long time to return to base values (1), it may be that these findings, indicative for MS, suggest that the initial ADEM triggered a process in this patient that later could lead to MS. The association of ADEM and MS is supported by the findings that certain patients may develop MS after an initial attack of ADEM (9, 10). Relapses in ADEM are rare, but should they occur, it has been recommended that the term “relapsing acute disseminated encephalomyelitis” be replaced by “relapsing disseminated encephalomyelitis” (5). The relapsing form of ADEM, however, may not be a separate entity from relapsing-remitting MS (5).
CT is insensitive to white matter changes compared with MR imaging and, therefore, may yield false-negative results in the presence of ADEM, despite widespread changes seen in MR imaging (1, 11–15). Also, there is typically a delay between the onset of the neurologic symptoms and the appearance of the ADEM-associated hypodense subcortical lesions sometimes showing ring-like enhancement seen on CT scans (16). In agreement with this, the initial CT scans performed on all four patients did not reveal any intraparenchymal changes in the brain. MR imaging scans, on the other hand, have proved to be the cornerstone in the diagnosis of ADEM. The ADEM-related changes in MR imaging scans include multiple hyperintense lesions seen in T2-weighted, non-contrast FLAIR, and PD MR imaging scans. The lesions may be large and confluent, occupying almost all of the white matter, but smaller lesions resembling those of MS are common (1). The lesions seen in ADEM are often located in the rim of occipital and parietal regions, including centrum semiovale. Hyperintense lesions in the brain stem and spinal cord are also frequently encountered (1, 8, 17). According to some authors, the lesions seen in ADEM are more asymmetrical than in MS (1, 17), and in this regard they may be distinguished from MS lesions (8). In AHEM, the lesions may later turn hemorrhagic (18), and this feature can be seen on MR scans of case 3.
Although ADEM typically is a monophasic illness, and the lesions would be expected to appear and mature simultaneously, new lesions may be seen on follow-up MR imaging scans (1). The lesions may or may not enhance with contrast medium, and a mixture of enhancing and non-enhancing lesions, depending on their age, may be seen (1, 17). This feature is also demonstrated in case 1: the first two lesions became inactive in the follow-up studies and did not enhance, whereas new, active lesions enhanced. The lesions usually show a rapid response to steroid therapy (12, 17), but they may also resolve gradually up to 18 months after the onset of the symptoms (1). In some cases, the lesions may resolve completely (15), but some of them tend to be permanent (1, 8, 15, 17). If the number of the lesions increases on long-term follow-up MR imaging scans, and especially if the patient develops new neurologic symptoms (5), then MS is the most likely diagnosis. In addition, MS is more likely if initial MR imaging detects typical demyelinating lesions, because initial MR imaging of brain is diagnostic in over 90% of MS patients (19). In our patient showing permanently increased IgG-index and pathologic IEF suggestive for MS, the first MR imaging scan showed no MS-like lesions. Hence, it seems to be unlikely that this patient suffered from her first MS relapse. On the follow-up MR imaging scans, the number of lesions did not increase; neither did they resolve. No new symptoms developed during an 8-month follow-up period. Whether this patient will later develop MS remains to be seen.
The resolution of the initial lesions has been suggested to parallel clinical recovery (1, 15, 17). On the other hand, it has been claimed that new lesions may appear during the recovery period (1). The latter appears to apply to the three patients described here who survived the disease. At the same time, when the number of new, active lesions appeared in MR imaging, the patients' condition continued to improve, and in fact, unlike one would expect, the appearance of multiple new lesions seemed to herald their recovery. Thus, the appearance of the MR imaging changes may actually have a negative correlation with the patient's condition. Furthermore, three of the four cases presented here showed a delay of about 1 to 6 weeks between the onset of clinical symptoms and the appearance of the lesions in MR imaging. Cases 1 and 2 were extensively evaluated with MR imaging performed on a weekly basis. For case 4, the initial MR imaging performed at day 4 did not show anything specific, but the control scan performed 2 months later indicated ADEM. The MR scans of this patient were not frequently evaluated, however, and so the exact length of the delay cannot be determined. The delay between the findings in neuroimaging studies and disease onset has been noted in a recent review (5). However, the delay cited by Stüve et al (5) mainly exists between the symptoms and CT findings (16, 20). In fact, the initial MR imaging scans have actually been reported to be indicative (8, 15, 17), and searches of current literature reveal only two cases in which the initial MR imaging failed to show any ADEM-related lesions (21, 22). In these studies, only conventional MR imaging techniques were used. The unconventional MR imaging techniques such as diffusion-weighted (DW) imaging, apparent diffusion coefficient (ADC), and magnetization transfer (MT) ratios may detect maturating demyelinating lesions in MS in areas termed as normal-appearing white matter before their appearance on conventional T2-weighted images (23–25). To date, there is only one report describing changes in ADC and DW imaging in ADEM (26). In this article, however, the predictive value of ADC and DW imaging was not addressed, because the initial T2-weighted scans of both the presented cases showed lesions characteristic to ADEM. To our knowledge, MT ratios have not been used in ADEM, and the prognostic value of MT imaging in detecting demyelinating lesions in MS has recently been questioned (27). Hence, it remains to be established whether changes in normal-appearing white matter detectable by novel MR imaging techniques precede their appearance on conventional MR imaging scans.
Conclusion
Here we demonstrated that in ADEM there may be a delay of over a month between the onset of symptoms and the appearance of lesions on conventional MR images. This delay should be taken into consideration if the clinical picture is suggestive for ADEM but the first MR imaging scans do not reveal any pathologic findings. Although no controlled trials exist in the treatment of ADEM, it can be presumed that timely diagnosis leading to prompt treatment may improve the outcome and prevent serious neurologic long-term consequences of this potentially fatal disease.
Acknowledgments
This study was supported by the Medical Research Fund of the Tampere University Hospital. We thank Drs. A. Hietaharju, O. Pammo, J. Mikkola, J.-P. Ahonen, and A. Sorri and professor H. Frey for their critical evaluation of the manuscript and for providing patient data. We also thank Ph. Lic. P Ryymin for his advice with the MR images.
Footnotes
1 This study was supported by the Medical Research Fund of the Tampere University Hospital.
2 Address reprint requests to Jari Honkaniemi, MD, PhD, Department of Neurology and Rehabilitation, University of Tampere, Box 607, 33101 Tampere, Finland.
References
- Received July 24, 2000.
- Accepted after revision February 9, 2001.
- Copyright © American Society of Neuroradiology