Abstract
BACKGROUND AND PURPOSE: Promontory testing is used for preoperative assessment of the auditory pathway before cochlear implantation. This method depends on patient cooperation and cannot be used in children or disabled persons. Promontory stimulation during functional MR imaging (fMRI) provides a new and objective method to test the integrity of the auditory pathway. To evaluate the method, we performed this prospective study in deaf adult patients.
METHODS: fMRI of the auditory pathway with electrical stimulation of the promontory was performed in 35 profoundly deaf patients, bilaterally in seven. For safe stimulation inside the MR environment, a specially designed nerve stimulator was used. We acquired nine sections parallel to the sylvian fissure by using an echo-planar pulse sequence (1.5 T). To evaluate the number of pixels in the auditory cortex, areas were counted and the minimum confidence level (pst value) was determined. The auditory pathway was called intact when the minimal pst value was 10−5 or when the minimal pst value was 10−4 in at least five activated pixels.
RESULTS: Images in 85% of patients reporting an auditory sensation showed activation of the contralateral auditory cortex. In the group of patients reporting no hearing sensation, images in 75% did not show activation.
CONCLUSION: This method can prove the intactness of the auditory pathway and help the surgeon in decision making before cochlear implantation. However, a negative finding should not be interpreted as indicating a nonfunctioning auditory pathway. Additional technical refinements and experience are needed to further improve this method.
Cochlear implants (CI) have proved to be effective and reliable assistive listening devices in postlingually, profoundly deaf adults, as well as in congenitally, profoundly deaf children (1, 2). As experience with cochlear implantation has increased, the selection criteria have been broadened, and patients with more difficult conditions such as additional disabilities or special etiologies of deafness are considered as candidates (1, 3, 4). Therefore, the preoperative diagnostic process becomes more important. In particular, an objective evaluation of the retrocochlear (central) auditory pathway is needed. CIs bypass the mechanical-electrical transduction in the hair cells of the inner ear, and they directly stimulate the auditory nerve via implanted electrodes. Although deafness involving the inner ear can be effectively treated with a CI, an intact retrocochlear auditory pathway is a basic requirement for a positive outcome with cochlear implantation.
Reliable preoperative assessment of the integrity of the central auditory pathway in adult patients is determined by performing the promontory electrical stimulation test (PT test) with a needle electrode. This is a subjective test that relies heavily on the patient’s sensation and cooperation during stimulation. The PT test is widely used, although it cannot be performed in small children or disabled patients (3, 4) because they are unable to cooperate adequately. An objective test is needed to evaluate those patients (1, 3, 4).
Developing a method for predicting the future benefit of cochlear implantation is a challenge for neurofunctional imaging. A safe stimulation device that can be used inside the gauss field of an MR system has been developed (5). The PT test with a needle electrode is used for activation during functional MR imaging (fMRI). Optical encoding of the signal intensity to minimize the length of electrical conductors and a radio-frequency-shielded transformation device are necessary to safely perform electrical stimulation during fMRI. All technical devices are manufactured with the strict avoidance of ferromagnetic materials (5, 6).
A number of fMRI studies of the auditory cortex have been conducted in healthy subjects (7–13). These have shown central activation with stimuli such as speech, words, nonsense syllables, pure tones, and music. Only a few groups have performed fMRI studies to try to show cortical activation in deaf patients (6). With different methods, such as proton emission tomography (PET) and electromagnetencephalography, data about brain plasticity in CI patients (14–15) or about the organization of the auditory cortex in congenitally deaf patients (16, 17) can be collected. None of these methods, however, provides an objective test of the integrity of the central auditory pathway. Designed as an objective test and presenting an approach different from the one presented here, the ear-canal electrode has been used for indirect stimulation of the cochlear nerve during fMRI; this has been used in five healthy subjects (18).
In the present study, we performed fMRI by using the promontory stimulating device in 35 deaf patients before cochlear implantation. The purpose was to determine the utility and predictive value of this test in establishing the presence of an intact central auditory pathway. Therefore, the activation results at fMRI were compared with the patients’ hearing and vibrotactile sensations during fMRI.
Methods
Patients
Thirty-five adult patients aged 20–74 years (mean, 40.1 years; 20 female and 15 male) were examined during a regular preoperative cochlear implantation examination. In seven patients, both ears were examined. A total of 19 right and 23 left ears were tested. Thorough audiological testing performed before fMRI included pure-tone audiometry; speech audiometry; and auditory brain stem response, otoacoustic emission, and promontory testing. PT test results, the patient’s history, and etiologies of deafness are listed in Table 1. An experienced ENT specialist (B.P.W.) rated the PT test results on the basis of the number of frequencies with a hearing sensation, the quality of the impression, and the detection of rhythm. A scale of 1–4 was used: 1 indicated a good result, clear constant hearing impression in at least four frequencies, with clear rhythm detection; 2, clear constant hearing impression in at least three frequencies with rhythm detection; 3, hearing impression in three frequencies with some nonauditory impression or minimal auditory fatigue; and 4, no clear hearing impression or massive auditory fatigue. CT scans revealed no cochlear malformations. Most patients (33 of 35) had a postlingual onset of deafness. In four patients, profound deafness occurred acutely (due to meningitis, trauma, or intoxication). The time elapsed since the occurrence of profound deafness was 0–25 years (mean, 5.2 years). Ten patients had minimal residual hearing.
History of Deafness and PT Test Results in 35 Patients with Profound Deafness
Data Acquisition
First, the patient was positioned on the MR table outside the MR suite. The stimulation device transforming the optical signal intensity into an electric signal was fixed to the patient’s head by using tight elastic bands. The signal was transmitted from the PT stimulator outside the MR suite to the stimulation device via optical fiber (5). After local anesthesia was induced, a PT needle made of tungsten was positioned at the promontory and connected to the stimulation device. A PT test was performed with 50, 100, and 200 Hz to find the optimal stimulation frequency and amplitude. The frequency with the lowest threshold and clearest sensation for the patient was chosen for use during fMRI by using an intensity that was 10–20% less than the patient’s discomfort level. The patient was than carefully moved into the MR unit without any changes in the setup. During fMRI, the PT stimulator was switched according to a stimulation pattern. After fMRI was performed, each patient was asked to describe any sensation during the examination inside the unit (Table 2). Any description of a periodical hearing sensation was accepted as a positive answer. This sensation was related to the acquired activation results to exclude differences due to the noise of the unit or possible technical defects during the examination.
Sensation During fMRI and Activation Data in 35 Patients with Profound Deafness
The fMRI was performed by using a 1.5-T MR system (Signa Horizon; GE Medical Systems, Milwaukee, WI). A gradient-echo echo-planar pulse sequence (TR/TE/NEX, 2700/72/1; matrix, 96 × 128; field of view, 22 cm; section thickness, 4 mm; no intersection gap) was used to acquire the functional data. We acquired nine axial images parallel to the sylvian fissure every 15 seconds, with a duration of 3 seconds. Total imaging time was 7 minutes 45 seconds, covering an activation paradigm with four off and three on periods, each with the same duration of 1 minute15 seconds. The paradigm began with an off period. Therefore, during each on or off period, five images of each section were acquired. The stimulation during the on period was continuous. T1-weighted images were acquired in the same location by using a spin-echo pulse sequence (420/10/2; matrix, 256 × 256; field of view, 22 cm; section thickness, 4 mm; no intersection gap).
Image Analysis
The postprocessing was completed on a SPARC workstation (Sun Ultra 5/10; Sun Microsystems, Mountain View, CA) by using the program Functool 1.0n (GE Medical Systems). Correlation maps were individually constructed for each patient and overlaid on anatomic T1-weighted images of the same location for visual inspection. The analysis of the data was performed by using three-criteria localization in primary and secondary auditory areas, the number of activated pixels and the confidence level of the correlation between the activation pattern, and the signal intensity time curve. The correlation was determined by means of linear regression and a Student t test. The confidence level was given as a pst value.
The activated pixels of the following areas were counted: 1) the primary auditory cortex (Brodmann areas [BA] 41 and 42) located in the medial two thirds of the transverse temporal gyrus (TTG) (19, 20); 2) the secondary auditory cortex (BA 22) located in the superior temporal gyrus and the temporal plane and including the Wernicke area for language perception (21), the medial geniculate body as the diencephalic relay station of the auditory pathway (22); and 3) the inferior third of the primary sensory cortex (postcentral gyrus), representing the sensory area of the face and including the external auditory canal and middle ear (22, 23). The anatomic examination was based on the Talairach Atlas and thorough anatomic descriptions of the functional auditory system (19, 22, 24).
To decide whether an examined auditory pathway could be called intact, we used two criteria to distinguish between the activated state and the nonactivated state. These were based on the results of the statistical analysis and the clinical data. Auditory cortex was considered activated if activated pixels were present in either the primary or secondary auditory cortex with a pst value less than 10−5. In addition, the finding of at least five pixels with a minimal pst value of 10−4 was also rated as activation of the auditory cortex. All other correlated pixels were rated as being nonactivated. The highly predictive value of the pst variable was determined by means of logistic regression. The results of the χ2 test suggested the cutoff values.
Statistical analysis was performed by using SPSS 9.01 (SPSS, Chicago, IL). A logarithmic regression and a χ (2) test were performed in the group of patients with auditory and nonauditory impressions during fMRI. For the latter test, the pixel numbers and pst values in the primary and secondary auditory cortex areas were included as variables. The patients with questionable auditory impressions were excluded from the statistical analysis. The term “questionable” was used to describe communication problems and the basic issue of whether a congenitally deaf patient can distinguish between a hearing sensation and a vibrotactile sensation at all.
Results
Among the patients with clear auditory impression during fMRI, 22 (85%) of 26 had activation in the area of the primary and/or secondary auditory cortex (BAs 41, 42, and 22) (Fig 1A–D). In the group of eight patients with no hearing impression during fMRI, no activation was found in six (75%). The exact distribution of all three groups is listed in Table 2 and Figure 2. Patients with a questionable impression are listed but not included in the statistical analysis.
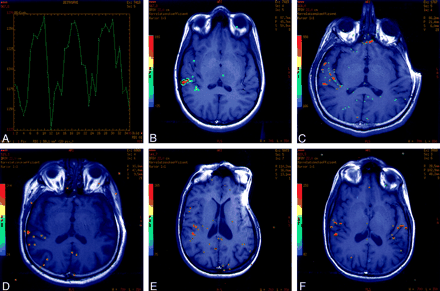
Activation in the area of the primary and/or secondary auditory cortex.
A, Signal intensity time curve.
B, Corresponding region of interest in the area of the auditory cortex.
C, Example of activation along the TTG (BAs 41, 42) with a pst value of P = 10−9.
D, Example of activation just above the cutoff pst value of P = 10−4.
E, Activation in the postcentral gyrus, primary sensory cortex (BAs 3, 1, 2)
F, Bilateral activation in the primary and secondary auditory areas
Distribution of activation in the three groups of patients with hearing and questionable and nonhearing impressions during fMRI.
Most activation was observed in the primary auditory cortex and the surrounding association areas (secondary auditory cortex). In these three cases, clearly activated pixels could be seen in the area of the medial geniculate body (mgb). The mgb represents a part of the central auditory pathway; it is the nucleus of the thalamus involved in this primary auditory pathway.
In three of six patients who reported a vibrotactile sensation during fMRI, activation in the lower third of the postcentral gyrus could be found (Fig 1E). This corresponded well to a vibrotactile sensation reported by these patients. A general evaluation of the postcentral gyrus was not performed, because the area was not completely covered in all examinations.
Bilateral activation was shown in two of the five patients (Fig 1F) in whom ipsilateral evaluation was possible. In all other examinations (37 of 42), the artifact due to the stimulation device prevented the evaluation of stimulation on the ipsilateral side.
The activation in the TTG (BA 21) was often found in the medial part, an area where high tones should be projected according to the tonotopic organization of the Heschl gyrus (11). This result could confirm the fact that the tone heard in PT tests is often described as being very high.
The results in the cases of two etiologies warranted separate consideration. The first etiology is the one of congenitally deaf patients. With the auditory deprivation in congenitally deaf patients, only incomplete development of the central auditory pathway system takes place. The central parts of the auditory system may be partly used by other sensory systems (1, 16). Of the three patients with congenital deafness in our study, one showed activation with slightly more activated pixels and a higher pst value in the primary area as compared with the secondary auditory area. The second etiology is one of meningitis. Meningitis can cause damage to the hair cells of the inner ear, as well as damage to the auditory nerve (1). In the two cases with deafness due to meningitis or meningoencephalitis, no activation could be identified. All of these patients reported a questionable or no auditory sensation.
Using the pst value of 10−5 or 10−4 and at least five activated pixels as a cutoff, the overall positive predictive value of the examination for a hearing sensation was 82%. Testing the zero hypothesis that hearing-nonhearing and activation are not related, the hypothesis is denied (P = .003). To determine which of the factors—localization (primary or secondary auditory cortex), number of pixels, and correlation coefficients—would have the best predictive value for a positive or negative hearing impression, we used logistic regression. Logistic regression was performed with pixel numbers and pst values as variables for both. Primary and secondary areas showed a significant influence on the pst value of the correlation coefficient in primary areas (P = .026) as well as for the correlation coefficient in secondary areas (P = .012).
Pixel numbers showed no significant influence on the primary (P = .164) or secondary areas (P = .248). When we assessed the lowest pst values of both areas together, the predictive value of this variable was highly significant for a hearing impression (P = .003). Therefore, the highest pst value of either primary or secondary cortex areas was used to define the cutoff between activated and nonactivated results.
Discussion
The purpose of this study was to determine if fMRI with promontory stimulation could help in identifying an intact auditory pathway in deaf subjects. Promontory stimulation resulted in activation of the auditory cortex in 85% of the group of patients with a clear auditory impression during fMRI (n = 26). Two other investigators performed fMRI of the auditory cortex with the purpose of conducting an objective auditory pathway evaluation with different stimulation devices (6, 18).
Bertezene et al (6) detected activation in three of seven cases by using a high electrical threshold just below the discomfort level comparable to the threshold used in our study (ie, 10–20% below the patient’s discomfort level). In the four remaining examinations resulting in no activation, a low electrical threshold just above perception level was used. This corresponds to the observation in the healthy subjects in the study by Strainer et al (7). They found that high-intensity tones activated significantly more pixels than lower-intensity tones at the same frequency (n = 10), but this finding could not be proved by Millen et al (8). This observation in healthy subjects and in CI candidates could also explain the nonactivation in the four false-negative ratings in our study. In addition, three patients with false-negative findings reported a strong vibration sensation during the examination; this might have influenced their perception. A phenomenon called descending inhibition is known from the sensory physiology (25). Therefore, we believe that the dominant different stimulus can influence the strength of the activation, especially regarding a weak auditory perception in chronically and profoundly deaf subjects.
Hofman et al (18) used a different stimulation device: an ear canal electrode. In a comparison of the needle electrode and the ear canal electrode in a clinical setting, Lesinski et al (26) revealed a number of undesirable effects with the ear canal electrode. A larger amount of vibrotactile sensations was found before the auditory impression. A significantly higher threshold level (>0.05) had to be used with the ear canal electrode, an effect that can be critical within the magnetic field. Discomfort levels were significantly higher with the needle electrode (26). Overall, auditory sensations in the clinical setting were found in 75.8%, as compared with 83.3%, with the needle electrode (26, 27). For the purpose of fMRI, the ear canal electrode causes no artifact (18); therefore, bilateral evaluation of the auditory cortex is possible. The transformation device, presently used with the promontory needle electrode, generated an artifact that allowed bilateral evaluation in only five patients. Because 80% of the auditory pathway is supposed to cross, the larger part of the activation could be expected on the contralateral side. Many investigators demonstrated a cortical asymmetry in blood flow and speech perception (28, 29). Other investigations involving PET demonstrated an equal contribution of activation in healthy subjects (15).
The differentiation between a vibrotactile and an auditory sensation in a PT test is difficult for prelingually deaf patients and sometimes postlingually deaf patients. Four patients had activation in the auditory cortex as well as in the sensory cortex. Three of them reported a vibrotactile sensation during fMRI, but with a questionable auditory sensation, if at all. One reported only an auditory sensation. With nine sections, it was often possible to cover the relevant area of the temporal lobe and also the lower third of the postcentral gyrus. This latter area represents the cortical distribution of the trigeminal system, including the sensory input from the facial and vagal nerves (22, 23). For this reason, the test is objective not only in terms of the auditory sensation but also in terms of the differentiation between a vibrotactile and an auditory impression, as shown in four patients.
Little is known about the organization and connectivity of the auditory cortex in congenitally deaf patients (4). Areas related to sign language, silent lip reading, and vibration have been shown, by means of different examinations, to be located in the auditory cortex area of congenitally deaf individuals (16, 17). Priming of the auditory pathway is believed to be completed around the age of 6 years (1, 2). According to this observation, cochlear implantation after this age often cannot meet expectations, and speech development does not occur as well as does in younger children (14, 15). Because all of our patients were adults, these facts can easily explain the missing activation in the auditory cortex in two of the three congenitally deaf patients. In the third patient, the auditory pathway was obviously intact, though further processing of the signal in the secondary auditory area did not show the same strength of activation. More examinations in congenitally deaf patients are necessary to further evaluate the development and priming of their auditory pathway.
The goal of this fMRI study was different from most fMRI studies performed. We wanted to prove that activation takes place in a certain predefined area in each individual patient. We did not intend to find all of the areas where a special stimulus would cause activation in general. Therefore, criteria for the level at which one would call an auditory pathway intact had to be defined. This definition was based on statistics but also depended on the localization and strength (number of pixels and pst-value) of the activation. The auditory pathway is believed to end primarily at the neurons in the area of the primary auditory cortex. Further connections transmit the signal intensity onto the secondary auditory cortex. In addition, some authors report core and belt projections, with the former ending directly in the area of the secondary auditory cortex (22). Directly and indirectly, an activation of the primary and/or secondary auditory areas indicate an intact auditory pathway.
Conclusion
Examinations with an activated auditory cortex virtually demonstrate an intact auditory pathway. This provided the widely accepted correlation between the blood oxygenation level–dependent effect and neuronal activation with regard to localization and time (30, 31). However, a negative examination finding that does not show activation cannot be interpreted as being indicative of a nonfunctioning auditory pathway. Technical problems or a very weak stimulus accounts for false-negative examinations. Electrical stimulation of the promontory during fMRI can therefore help in making a clinical decision before a cochlear implantation, although a negative result must be considered with great care. Further technical refinements and experience with more patients, especially congenitally deaf patients, are needed to improve the predictive value of this method.
Acknowledgments
We thank Dr. rer nat Hartmut Herrmann for statistical support, Gabriele Richardson for language support, Dr med Martin Bokemeyer for his helpful comments on the manuscript, and Christa Karre and Dagmar Weiβkopf for assistance with patient coordination.
Footnotes
Supported by DFG No. BE 768/2–1.
Presented in part at the Joint International Conference & Symposium of the American and the European Society of Head & Neck Radiology, Washington, DC, 2000.
References
- Received July 17, 2000.
- Accepted after revision August 27, 2002.
- Copyright © American Society of Neuroradiology