Abstract
BACKGROUND AND PURPOSE: An accurate diagnosis of a parotid gland mass is essential for adequate management. We determined the clinical efficacy of USCNB in diagnosing parotid gland masses by using cutting needles of different bores.
METHODS: We reviewed records for 40 benign and 13 malignant parotid lesions. USCNB was performed by using 14–20-gauge needles (mean, 16.6 gauge) with one to five (mean, 2.43) passes and a 15-mm throw or specimen notch. Final diagnoses were established on the basis of surgicopathologic results in 31 cases, and on the basis of histopathologic analysis of biopsy specimens, clinical data, and/or imaging studies in 22, with a follow-up of 12.2–77.5 months (mean, 33.6 months).
RESULTS: Compared with surgicopathology, USCNB had a sensitivity of 83%, a specificity of 100%, and an accuracy of 97% in providing specific tissue diagnoses and in differentiating malignant from benign masses. Its positive and negative predictive values were100% and 96%, respectively, in diagnosing malignancy. One patient (2%) had a local hematoma without sequela after surgical removal of a Warthin tumor. Core biopsy results were completely concordant with surgical findings in 30 (97%) of 31 cases.
CONCLUSION: USCNB is a safe and efficient diagnostic procedure with an accuracy of 97% in the pathologic diagnosis of parotid masses. It can be performed in an outpatient clinic and enables specific tissue diagnosis to obviate intraoperative frozen biopsy and unnecessary surgery. An 18-gauge needle is sufficient for accurate and specific tissue diagnosis of parotid masses.
The presence of a mass in the parotid gland is a diagnostic and therapeutic challenge. A non-neoplastic lesion, benign neoplasm, or malignant neoplasm can cause such masses. Fine-needle aspiration biopsy (FNAB) has been advocated as a first-step procedure after history taking and physical examination for the evaluation of a mass or enlargement of the parotid glands (1). However, in a series of 151 cases, FNAB findings were nondiagnostic or inconclusive in 18% (2), and its sensitivity was 53–79% for malignancy and 76–92% for benign neoplasms (1, 3–5). Aspiration did not provide an adequate specimen for diagnosis in 9–12% of all cases and in 14% of malignancies (5, 6). In most cases, a specific diagnosis of salivary gland carcinoma cannot be made with FNAB (7).
Improvement in the accuracy of the preoperative diagnosis of a parotid gland mass is essential to avoid unnecessary surgery, to obviate frozen section biopsy, and to select adequate management (8–10). A prospective randomized study of three sizes of core-cutting needles for renal transplant biopsy revealed that the diagnostic usefulness was higher for 14- or 16-gauge needles than with 18-gauge needles (11). To our knowledge, some investigators published articles regarding usage of 18 or 20 gauge cutting needles in ultrasonography-guided core-needle biopsy (USCNB) of the parotid gland in 1999 (12) and 2002 (13). Before that report (12), we sometimes used larger needles of up to 14 gauge for USCNB of parotid gland masses because of a concern about obtaining insufficient tissue samples. Since 2000, 18- or 20-gauge needles have been more commonly used. The purpose of our study was to describe our experience regarding the safety and clinical efficacy of USCNB with needles of different sizes in the management of 53 parotid gland lesions involving the superficial lobe.
Methods
During the 5 years and 7 months from April 1997 to November 2002, 53 patients with palpable parotid masses underwent USCNB in our hospital. They included 35 men and 18 women aged 17–81 years (mean, 50.9 years). The maximal diameter of the masses was 1.4–8 cm (mean, 2.9 cm) on sonograms.
Before the procedures, the indication, risks, benefits, and possible complications of the procedures were well explained to the patients or their families. The biopsy procedures were performed on an outpatient basis with the patient in the lateral decubitus position. The skin was sterilized and locally anesthetized with 1% lidocaine (Xylocaine; Astra Pharmaceutical; Westboro, MA). All patients were asked to compress the puncture biopsy site afterward and were observed for half an hour before leaving the examination room.
All lesions were well depicted in the superficial lobe of the parotid gland. The biopsy procedures were done under the guidance of a real-time scanner (128X, Acuson, Mountain View, CA; Sonoline Elegra, Siemens, Issaquah, WA) by using a 7- or 7.5-MHz linear transducer and a cutting needle (Bard Magnum, Covington, GA; Temno, Allegiance Healthcare Corporation, McGaw Park, IL). A freehand technique, a Bard Magnum gun with a 15-mm throw or a cutting needle with a 15-mm specimen notch were used. The lesion at the superficial portion was chosen as the biopsy site. We attempted to keep the angle between the biopsy needle and the skin surface less than 45° to avoid injury to the deeper structures, such as the parotid duct or facial nerve. One (n = 1), two (n = 32), three (n = 17), four (n = 2), or five (n = 1) passes (mean ± standard deviation [SD] = 2.43 ± 0.69) were made by using a 20-gauge (n = 1), 18-gauge (n = 20), 16-gauge (n = 26), or 14-gauge (n = 6) cutting needle (mean gauge ± SD = 16.60 ± 1.39). The estimated volume of acquired tissue ranged from 18 to 262 mm3 (mean ± SD = 70.82 ± 39.21 mm3) (Table).
Final diagnoses of 53 parotid lesions, patients undergoing surgery, needle sizes, passes, and volume of acquired tissue
The specimens were put in a small jar with 10% formalin solution for pathologic study. Since the bores of the cutting needle for parotid biopsy were not fully well established in the literature, the needle size was chosen mainly according to each radiologist’s preference.
The Table summarizes the final diagnosis, number of patients undergoing subsequent surgery, sizes of the cutting needles, number of passes, and volumes of acquired tissue. Of 53 patients, 31 underwent subsequent surgery 2–280 days (mean, 57.1 days) after core biopsy. Final diagnoses were established on the basis of subsequent surgical and pathologic findings in 31 cases and on histopathologic findings of the biopsy specimens, clinical data, and/or imaging studies in another 22 cases, with a follow-up of 12.2–77.5 months (mean, 33.6 months).
Using a 2 × 2 contingency table, we calculated the diagnostic sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) of USCNB in differentiating malignancy from benign lesions against the final diagnoses based on surgicopathology in 31 cases. The 95% confidence interval (CI) was provided to compare our finding with other studies.
Results
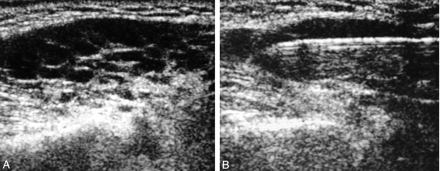
The Table summarizes the final diagnoses of the 40 benign and 13 malignant cases, and Figure 1 shows images from one patient (Figs 1). Of 31 patients who underwent surgery after USCNB, results of core biopsy were completely concordant with those of surgicopathology in 30 (97%). Results in only one patient (3%) were not fully concordant between biopsy and surgery. In this case, core biopsy revealed an organized abscess, but surgicopathology revealed an additional finding of clear cell adenocarcinoma. Twenty-two (26%) of 53 patients did not undergo surgery; these patients had five lymphomas, two metastatic carcinomas, seven nonneoplastic benign lesions, and eight benign neoplasms (four pleomorphic adenoma, three Warthin tumors, and one oncocytoma). Four of these patients died 0.3–33 months (mean, 13.6 months) later from causes unrelated to the biopsy procedure. The remaining 18 patients did not have local recurrence after 12.2–77.5 months (mean, 38 months) of follow-up. Only one patient (2%) had a complication of hematoma, but this was without sequela.
A 69-year-old man with low-grade lymphoma of the parotid gland.
A, Hypoechoic mass with linear echogenic foci within the tumor.
B, Core biopsy of the mass with a cutting needle passing through the tumor.
On the basis of the surgical and pathologic results in 31 cases, USCNB had a sensitivity of 83% (95% CI: 40.4%, 99.6%), a specificity of 100%, and an accuracy of 97% (95% CI: 80.3%, 99.9%) in providing specific tissue diagnoses and in differentiating benign masses from malignant masses. USCNB had a PPV of 100% and a NPV of 96% (95% CI: 77.2%, 99.9%) in diagnosing malignancy. Because of the small sample size, the accuracy of core biopsy could not be statistically correlated with the size of the cutting needle, the number of passes, and the volume of biopsy tissue acquired.
Discussion
US is characterized by its ease of manipulation, capability of multiplanar scanning, noninvasiveness, and portability. It is one of the noninvasive imaging tools used to evaluate parotid masses, especially those in the superficial lobe (8–14). However, in contrast to CT and MR imaging, US cannot show deeper lesions and their associated medial extension because of obscuration by the mandible, especially with large lesions (15). Fortunately, most parotid lesions in this series with a mean diameter of 3 cm involved the superficial part, as described previously (16, 17).
Surgical excision or enucleation is the treatment of choice for Warthin tumor (9, 10) and was performed in 12 of 15 patients. Parotidectomy was performed in 11 of 15 patients with pleomorphic adenoma. Of 53 patients, one underwent surgical excision of a pleomorphic adenoma 5 months before clinical manifestation of parotid mass. However, the recurrent mass was pathologically proved to be fibrosis; therefore, unnecessary surgery was avoided. Sialadenitis (found in two patients) is usually treated with antibiotics, whereas an inflammatory lesion or lymphoid hyperplasia (found in three and one patients, respectively) are usually observed and conservatively treated. All six patients with lymphoma underwent chemotherapy and/or radiation therapy. Four of six patients with carcinoma received extensive surgery or even lymph node dissection (10, 12, 18, 19).
Core-Needle Biopsy and FNAB
The advantages of FNAB include decreased tissue damage, no need for anesthesia, and repeated sampling when specimens are insufficient after immediate assessment at the examination site. However, we suggest that a patient with a parotid gland mass undergo core-needle biopsy rather than FNAB because USCNB is similar to FNAB in that it can be performed on an outpatient basis. Moreover, the establishment of a definite pathologic diagnosis with USCNB may obviate unnecessary surgery or time-consuming frozen biopsy during surgery. Our series revealed that USCNB had a sensitivity of 83% (95% CI: 40.4%, 99.6%), a specificity of 100%, an accuracy of 97%, a PPV of 100%, and an NPV of 96% in diagnosing malignancy. These results were comparable to those of two previous reports (12, 13). Only one complication with hematoma was encountered in this series. Furthermore, on the basis of histopathology, long term follow-up of clinical data and imaging studies, our study showed that USCNB had a sensitivity of 98%, a specificity of 100%, and an accuracy of 98% in establishing specific tissue diagnoses.
Final diagnoses of core biopsy and surgery were discordant in only one patient. Before core biopsy provided a diagnosis of organized suture abscess at our hospital, the patient had received parotid surgery because of a mass at another local clinic. Surgicopathologic analysis done 3 months after core biopsy in our hospital revealed an additional finding of clear cell carcinoma despite organized abscess. Late recurrence of the tumor or insufficiency of the specimen might partially account for the incomplete diagnosis from core biopsy.
Complications and Study Limitations
The main objections to core biopsy of the parotid gland are the risk of facial nerve injury and tumor seeding along the needle tract. Before the report about the sufficiency of 18-gauge needles was published (12), 14- or 16-gauge needles were used more frequently in our institute. From our experience, the use of 14- or 16-gauge needles with up to five passes allowed us to obtain larger core samples for immunohistochemistry without sequela or facial nerve injury. Of 53 patients, only one patient (2%) who had a Warthin tumor had hemorrhage after three passes with a 16-gauge needle. The hemorrhage was probably related to the cystic components of the tumor, which occurs in 67–93% of cases (8, 9). However, no sequela was observed after surgical removal of the Warthin tumor. In this series, there was no infection, facial nerve palsy, or recurrence due to seeding of cancer at the needle tract.
Injury to the facial nerve with subsequent paralysis and facial deformity is a critical issue with core-needle biopsy of parotid gland lesions (12, 13). Within the parotid gland, the facial nerve is indistinguishable on imaging studies, but it can be traced according to the identification of the parotid duct (15, 20). The parotid duct is a landmark for localizing the mass superficial or deep to the facial nerve.
To avoid injury to facial nerve, we first suggest that USCNB must be monitored with real-time US, though the facial nerve cannot be seen. Second, the anterior tip of the needle should be confined to the mass before and after cutting. Third, penetration of the needle deep into the gland must be avoided. To comply with these suggestions, we used a 15-mm throw for a biopsy gun or a cutting needle with a shorter specimen notch. Around 80–90% of parotid masses are located in the superficial lobe (16, 17, 20); therefore, it is safe to perform USCNB if one follows these suggestions.
In 11 of our 15 patients with pleomorphic adenoma, the tumor was surgically excised after USCNB. Biopsy might disrupt the tumor capsule. Fortunately, none of our patients with pleomorphic adenoma who underwent surgery had tumor recurrence during follow-up of 28.9–76.5 months (mean, 48.6 months). The recurrence of pleomorphic adenoma is not related to the size or location of tumor, but rather, with the microscopic presence of pseudopodia (21).
The risk of seeding of tumor cells along the needle tract varies according to the organ or size of needle used, with rates of up to 12% after FNAB, 24% after needle biopsy, and only 0.02% for abdominal tumors (22–24). The incidence of implantation and growth seems to depend on at least three factors: the cytokinetic characteristic of the seeded cells, the fertility of the tissue in which the tumor cells are seeded, and the number of seeded cells and the amount of stroma (25). The larger the bore of the core needle, the higher the possibility of tumor seeding, probably because larger bore needles allow the aspiration of sufficient stroma, which is essential for the survival of malignant cells (24–26).
This study had several limitations. First, we used larger needles of up to 14 gauge; however, the use of smaller-bore or 18-gauge needles was not reported until the end of 1999 (12), and many of our biopsy procedures with 14- or 16-gauge needles were done before 1999. Second, this is a retrospective rather than prospective study; therefore, the size of cutting needle was used according to each radiologist’s preference. Third, the actual volume of biopsy tissue acquired in each case might have been smaller than the estimated volume. Fourth, it may be inappropriate to conclude that all of our patients with malignancy were free of tumor seeding after biopsy, though excision of the needle track at the time of definitive surgery (22), chemotherapy in cases of lymphoma, and monitoring of biopsy under real-time US might have prevented tumor seeding. Tumor implantation along the needle tract is rarely reported; cases include cancer of the parotid and thyroid glands, lungs, and other organs (22, 25). The incidence of implantation after perineal prostatic needling is calculated to be 0.34% over 6000 needle procedures in 12 published series, and the average interval between the needle procedure and the clinical appearance of an implanted nodule is 13 months, with a median interval of 8 months (25).
Conclusion
USCNB is a safe, simple, and efficient diagnostic procedure with an accuracy of 97% in diagnosing parotid masses. It can be performed in an outpatient clinic, and it enables a specific tissue diagnosis to obviate intraoperative frozen biopsy and avoid unnecessary surgery. The different bores of needles yield similar results; therefore, an 18-gauge cutting needle is sufficient to make an accurate and specific tissue diagnosis.
References
- Received December 2, 2003.
- Accepted after revision February 14, 2004.
- Copyright © American Society of Neuroradiology