Abstract
BACKGROUND AND PURPOSE: In severe carotid stenosis, blood supply via the poststenotic internal carotid artery (ICA) and collateral pathways determine cerebral perfusion. We investigated whether poststenotic flow on transoral carotid ultrasonography (TOCU) is predictive of cerebral hemodynamics.
METHODS: Eighty-eight patients with unilateral carotid stenosis underwent TOCU to analyze blood flow velocity and poststenotic diameter of the extracranial ICA. Intracranial collaterals and cerebral hemodynamics were assessed with selective angiography and single photon emission CT.
RESULTS: Poststenotic diameter (P < .0001) and velocities (peak systolic velocity [PSV], time-averaged mean velocity [TMV], end-diastolic velocity [EDV]; all P ≤ .009) decreased with stenotic severity. Poststenotic diameter was correlated with PSV (r = 0.36, P = .0005), EDV (r = 0.32, P = .002), and TMV (r = 0.39, P = .0001). Poststenotic flow was significantly lower with collateral pathways than without pathways (P ≤ .02) and significantly decreased as the number of the collaterals increased (P < .0001). Flow <5 mL/s indicated collaterals (81% sensitivity, 80% specificity). When flow was <5 mL/s, the asymmetry index in the middle cerebral artery (MCA) territory was significantly low during rest (P = .003) and after acetazolamide challenge (P = .006). Poststenotic flow velocity was associated with baseline (P = .007) and postacetazolamide (P = .0009) MCA asymmetry indexes.
CONCLUSION: Poststenotic ICA flow measured with TOCU reflects collateral flow and cerebral hemodynamics in patients with severe carotid stenosis. This technique may provide new parameters for screening patients with hemodynamically significant carotid stenosis.
Atherothrombotic stenosis originating in the internal carotid artery (ICA) is a common cause of ischemic stroke in the carotid territory, and the risk of stroke increases with the severity of carotid stenosis (1–3). It is reported that impaired cerebrovascular reactivity is predictive of cerebral ischemic events in patients with carotid disease (4–10). On the other hand, it is also well recognized that the simple presence of carotid stenosis does not predict the presence or degree of hemodynamic compromise in the distal cerebral circulation (11, 12). Blood flow via poststenotic ICA and intracranial collaterals determines cerebral blood flow in these patients. However, poststenotic flow and its relation to collaterals and cerebral hemodynamics are not fully understood.
Reports have shown the diagnostic capacity of various techniques for detection of hemodynamically significant stenosis, such as the evaluation of signal intensity on ophthalmic artery (OA) color duplex scanning (13), use of carotid Doppler criteria (14), assessment of transcranial Doppler (TCD) findings (15, 16), the combination of MR spectroscopy and MR angiography (MRA) (17), and the combination of TCD and MRA (18). The detection of reduced poststenotic flow through the carotid stenosis is direct evidence of hemodynamically significant stenosis. However, conventional methods such as ultrasonography and MRA cannot reliably measure poststenotic flow. Some have reported (19) that transoral carotid ultrasonography (TOCU), pioneered by Yasaka et al (20), provides additional information on the characteristics of the distal ICA, even in patients with near-occlusion of the carotid artery. In the present study, we used this noninvasive alternative of TOCU to detect poststenotic signals and investigate their relationship to collaterals and cerebral hemodynamics.
Methods
Patients
Subjects were recruited from patients who had carotid stenosis originating in the ICA and who were scheduled to undergo carotid endarterectomy at the National Kyushu Medical Center, Japan. Patients who had obstructive lesions in intracranial cerebral, contralateral carotid, or vertebrobasilar artery exceeding 50% (as determined on angiography) were excluded. Those with a history of major stroke in the ICA territory confirmed on MR imaging and considered to affect interpretation of their cerebral hemodynamics were also excluded. Eighty-eight patients were selected for this study (73 men, 15 women; mean age ± SD, 68.6 ± 7.0 years). Three patients had undergone carotid angiography alone and were excluded from the analysis of collateral pathways. The present study was performed with the informed consent of all subjects to be examined with various neuroradiologic tests within a month before carotid endarterectomy.
Conventional Ultrasonography
Ultrasonography was performed by using a color-coded duplex ultrasonographic device (ATL HDI 5000; Hitachi, Tokyo, Japan). A 5–10 MHz sonography beam was used for conventional carotid imaging. Conventional carotid ultrasonography was performed by a neurosonographer blinded to the patients’ information. Percentage stenosis was measured during conventional ultrasonography by dividing the luminal area by the total vascular cross-sectional area at the point of greatest stenosis. The average degree of stenosis evaluated on conventional ultrasonography was 87% ± 11. Peak systolic velocity (PSV), end-diastolic velocity (EDV), and time-averaged mean velocity (TMV) were measured and corrected with the incident angle. Measurement was performed three times, and the mean value was calculated.
TOCU Study
The poststenotic portion of the extracranial distal ICA was investigated with TOCU. TOCU was performed by using a 5–9 MHz convex-array transducer. The methods for TOCU have been described previously (19). An experienced examiner (K.K.) performed all examinations without knowledge of the degree or side of the stenosis. The properties of the blood vessel were evaluated by B-mode study and by color flow imaging. PSV, EDV, and TMV were measured and corrected with the incident angle. Measurements were performed three times, and the mean value was used. Blood flow was estimated as follows: diameter2 × π × (TMV/4). Sensitivity and specificity of poststenotic blood flow in predicting collateral pathways were evaluated. The positive and negative predictive values were also calculated.
Single Photon Emission CT
An evaluation of the cerebral circulation was done by using single photon emission CT (SPECT). The apparatus used for SPECT was a two-head SPECT system (Prism 2000 XP; Picker, Cleveland, OH), and the tracer was a technetium-99m ethyl cysteinate dimer. Two regions of interest were symmetrically located over the cerebral hemispheres. The sizes and shapes of the region were designated to include the territory of the middle cerebral artery (MCA) and predefined by using the outline of a normal cerebral hemisphere. We evaluated the asymmetry of cerebral blood flow by using the asymmetry index, which was calculated as the difference between hemispheres as a ratio of the mean, as follows: asymmetry index = [(I − C)/(I + C)] ×2, where I and C refer to the maximal cerebral count value on the side ipsilateral to the carotid stenosis and that on the contralateral side, respectively. The asymmetry index was calculated before and after the intravenous administration of acetazolamide (0.017 g/kg).
Angiographic Assessment
Conventional selective angiography was performed in all patients. A neuroradiologist reviewed the angiograms blinded to all information. The degree of carotid stenosis on angiography was assessed by using the same method as that used in the North American Symptomatic Carotid Endarterectomy Trial (1, 3). The mean degree of stenosis was 77% ± 15. To assess the presence of collateral circulation, biplane views were examined. Definite filling of the anterior communicating artery (ACoA), posterior communicating artery (PCoA), and OA was considered to demonstrate the presence of collaterals. The presence of leptomeningeal collaterals (LM) was defined as retrograde MCA flow reaching the surface of the insula, as based on a previous study (21).
Statistical Analysis
Regression analysis was performed to examine relationships among poststenotic parameters, carotid stenosis, and cerebral hemodynamics. We analyzed the difference in poststenotic parameters, carotid stenosis, and asymmetry index by using the t test. We also used one-way analysis of variance (ANOVA) to compare each angiographic finding as a nominal variable with poststenotic parameters in the distal ICA as a continuous variable. A post-hoc Bonferroni test was done to detect the differences. Multiple regression analysis was performed to examine the possible association of the various parameters in the poststenotic portion with cerebral hemodynamics. Regression models included the potential predictive variables. These variables are poststenotic flow and diameter of the distal ICA and the presence of collateral pathways. A P value of >.05 was considered to indicate a significant difference. Values were expressed as the mean ± SD.
Results
Carotid Stenosis and Change in Poststenotic Distal ICA
Poststenotic PSV, TMV and EDV measured with TOCU were 66.5 ± 26.3, 37.8 ± 14.6, and 24.0 ± 9.2 cm/s, respectively. No significant stenosis was observed at the level of poststenotic portion where TOCU could approach. Prestenotic or intrastenotic ICA flow velocity measured with conventional ultrasonography were as follows: PSV, 158.6 ± 107.7 cm/s; TMV, 90.2 ± 70.6 cm/s; EDV, 58.4 ± 49.1 cm/s. Poststenotic flow velocities were correlated with the cross-sectional luminal area (quadratic correlation; PSV, r = 0.32, P = .009; TMV, r = 0.35, P = .004; EDV, r = 0.35, P = .004). The poststenotic extracranial ICA measured on TOCU was significantly narrower (3.6 ± 0.7 mm) than that of contralateral normal ICA at identical portions (4.0 ± 0.6 mm; P < .0001, paired t test). It was correlated with the severity of carotid stenosis (quadratic correlation; r = 0.54, P < .0001). Poststenotic diameter of the ICA was associated with poststenotic PSV (r = 0.36; P = .0005, linear regression), EDV (r = 0.32, P = .002), and TMV (r = 0.39, P = .0001).
Poststenotic Blood Flow and Collateral Pathways
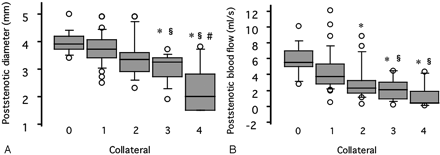
Blood flow and diameter of the poststenotic ICA were significantly lower in patients having collateral flow via the ACoA, PCoA, OA, or LM than those without such collaterals (Table 1). Moreover, the diameter and blood flow in the poststenotic ICA significantly decreased as the number of the collateral pathways increased (P < .0001, ANOVA) (Fig 1).
Variables in the poststenotic portion of the ICA plotted against the number of the collateral pathways. Asterisk indicates P < .005 versus patients with no collaterals; section symbol, P < .005 versus patients with one collateral; andnumber sign, P < .005 versus patients with two collaterals.
A, Poststenotic diameter.
B, Poststenotic blood flow.
Poststenotic parameters related to type of collateral pathway
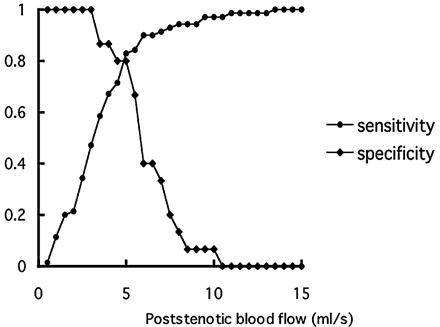
The best cutoff of poststenotic blood flow for detecting collateral pathways was established with a receiver operating characteristic curve. Poststenotic blood flow <5 mL/s was helpful in detecting collateral flow (Fig 2). Sensitivity and specificity were 81% and 80%, respectively, and the positive and negative predictive values were 95% and 50%, respectively.
Sensitivity-specificity curve. Optimal threshold value of poststenotic blood flow for predicting collateral pathways is 5 mL/s.
Poststenotic Variables and Ipsilateral Cerebral Blood Flow
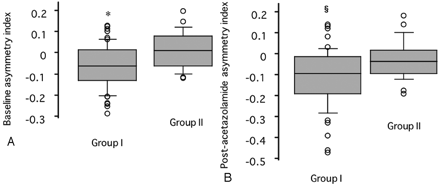
Asymmetry indices before and after acetazolamide provocation were −0.03 ± 0.10 and −0.08 ± 0.13, respectively. Poststenotic blood flow showed a weak but significant association with the asymmetry index before (linear regression, r = 0.25; P = .03) and after (r = 0.32; P = .004) acetazolamide challenge. The baseline asymmetry index in the cerebral hemispheres was significantly lower in patients with poststenotic blood flow <5 mL/s (−0.05 ± 0.10) than in patients with blood flow ≥5 mL/s (0.02 ± 0.09; P = .003, unpaired t test) (Fig 3A). Similarly, the postacetazolamide cerebral asymmetry index was significantly lower in patients with poststenotic blood flow <5 mL/s (−0.10 ± 0.14) than in patients with poststenotic blood flow ≥5 mL/s (−0.02 ± 0.09; P = .006, unpaired t test) (Fig 3B).
Baseline and postacetazolamide asymmetry index in the MCA territory plotted against poststenotic blood flow. Group I indicates patients with flow <5 mL/s; Group II, patients with flow ≥5 mL/s; asterisk, P < .005; and section symbol, P < .01 versus group II.
A, Before acetazolamide.
B, After acetazolamide.
Multiple regression analysis demonstrated a strong relationship between blood flow velocity (i.e., TMV) in the poststenotic extracranial ICA and the baseline asymmetry index (Table 2). This relationship was independent of other potentially predictive variables, including the poststenotic diameter, OA flow, and collaterals. Multiple regression analysis also demonstrated that the asymmetry index after the acetazolamide challenge was associated with poststenotic blood flow velocity (Table 2). When PSV or EDV was adopted as an independent variable instead of TMV, they were also significantly associated with the cerebral asymmetry index.
Results of multiple regression analysis
Discussion
Carotid Stenosis and Poststenotic Blood Flow
The extracranial ICA distal to severe stenosis is often narrowed or collapsed on angiograms (22, 23). An ICA-to-common carotid artery (CCA) ratio measured on angiography has been the criterion standard for the assessment of poststenotic narrowing (22). However, it is still difficult to identify minor degrees of narrowing in individual patients during angiography because of variations in the normal ICA-CCA ratio. Moreover, the ICA-CCA ratio possibly differs from that observed on ultrasonography in patients with severe stenosis (19). The assessment of the distal ICA by using conventional duplex carotid ultrasonography is technically difficult. One report has shown that the distal ICA could be visualized with a 3.5-MHz convex probe (24), but the observation range is still limited by the conventional approach. Moreover, the stenosis is mostly located in the origin of the ICA and higher degrees of luminal narrowing produce intrastenotic flow increase and poststenotic turbulence. As a result, blood flow velocity measured with conventional carotid ultrasonography is altered depending on the degree of stenosis. On the other hand, TOCU appears to be useful in measuring poststenotic flow without such influence. The present study demonstrated that poststenotic parameters could be obtained accurately by using TOCU. We believe this is the first study to elucidate the importance of poststenotic parameters in cerebral hemodynamics in patients with carotid stenosis.
The extent to which blood flow is reduced across the stenosis and how much cerebral perfusion pressure consequently decreases are unclear. Intraoperative measurements in the cervical carotid artery distal to a stenotic lesion demonstrate that reductions in flow occur when the luminal diameter is reduced more than 75% in diameter or 94% in area (25). Rothwell and Warlow (22) assessed poststenotic narrowing with an ICA-CCA ratio and showed that the ICA begins to narrow when the degree of stenosis reaches 70% with the European Carotid Surgery Trial (ESCT) method. In the present study, TOCU revealed that the poststenotic blood flow is highly reduced and the poststenotic lumen is collapsed when carotid stenosis is severe. Narrowing of the poststenotic lumen is possibly due to low intramural pressure caused by reduction of flow across the severe stenosis, since poststenotic diameter is associated with poststenotic flow.
Poststenotic ICA Flow and Cerebral Hemodynamics in Relation to Collaterals
Collateral flow via the circle of Willis is considered the primary collateral pathway. Reversed flow through the OA and blood flow via LMs are recognized as secondary collateral pathways (12, 26–31). Pial collateralization has been considered to be most consistently correlated with stage 2 hemodynamic compromise (11). However, one study showed no correlation between anatomic findings on angiographic studies and increased oxygen extraction fraction in patients with carotid occlusion (21). Therefore, the effect of collateral supply on cerebral hemodynamics is still controversial. Stenosis originating in the ICA may affect the blood flow through the OA and intracranial collaterals. In the present study, poststenotic blood flow estimated with TOCU was significantly low, irrespective of the type of collateral in patients who had angiographically identified collateral flow. Moreover, the poststenotic blood flow significantly decreased with the development of collateral pathways. Multiple regression analysis demonstrated that poststenotic blood flow velocity alone is associated with cerebral hemodynamics and that neither the presence of collaterals nor the degree of carotid stenosis is independent of cerebral hemodynamics. The reduction of blood flow velocity across the stenosis may be the important determinant of ipsilateral cerebral blood flow that results in the development of collaterals. The other possibility is that collaterals may affect the poststenotic flow of the ICA. Decreased poststenotic flow may provide evidence of collateral pathways and, consequently, disturbed cerebral perfusion.
TOCU Evaluation of Carotid Near-Occlusion
Rothwell and Warlow (22) studied near-occlusion by using carotid angiograms from the ECST and defined the term poststenotic narrowing and collapse of the ICA as an ICA-CCA ratio <0.42. Poststenotic narrowing may help in identifying patients at a particularly high risk of stroke who require urgent endarterectomy, since compromised cerebral blood flow may be an important causal factor in patients with a symptomatic lesion in the carotid artery. On the other hand, results of several studies suggest that poststenotic narrowing is protective in ischemic stroke, because blood flow distal to the stenosis may be insufficient to carry emboli to the brain (3, 22, 23). Some have reported that TOCU enables us to measure luminal diameter and flow velocity in poststenotic ICA, even in patients with near-occlusion (32). This technique verified that poststenotic narrowing did occur and that poststenotic blood flow decreased when the cross-sectional luminal area was less than a certain level. Thus, TOCU may be useful in understanding near-occlusion, and it may provide new insights into the medical and surgical options for treatment.
Limitations
In the present study, flow volume was calculated by using the product of flow velocity and the area, with an assumption that symmetric, parabolic, laminar flow is present. However, there are potential errors in detecting not only diameter but also flow velocity due to angle correction, turbulent flow, and nonparabolic flow. Another limitation is in the quantification of cerebral blood flow. Cerebral perfusion was evaluated by using asymmetry index measured with 99mTc ethyl cysteinate dimer SPECT. Instead, PET is preferred to assess the cerebral hemodynamic state.
Conclusion
Despite these limitations, poststenotic flow measured with TOCU was indicative of intracranial hemodynamics in patients undergoing carotid endarterectomy. Further study is needed to elucidate the importance of TOCU study in the evaluation of intracranial cerebral perfusion in patients with carotid stenosis.
Footnotes
Supported by Research Grants for Cardiovascular Diseases (12A-2, 12C-10, and 14A-3) from the Ministry of Health, Labour and Welfare of Japan.
References
- Received February 6, 2004.
- Accepted after revision April 14, 2004.
- Copyright © American Society of Neuroradiology