Abstract
Summary: Embolization of type I perimedullary spinal arteriovenous fistulas (AVFs) can be difficult, because of tortuosity and the small diameter of the feeder and distal location of the fistula site. The 1.5F flow-directed catheter in conjunction with a hydrophilic guidewire has been used in fistula embolization with cyanoacrylate glue for spinal vascular malformations at our institution. This combination has improved our success rate in achieving superselective catheterization of the fistula. Thus, 4 of 5 patients with type I perimedullary AVFs could be cured with this technique. Like type II and type III perimedullary AVFs, the endovascular approach may also be the first line of treatment in type I perimedullary spinal AVF.
Perimedullary arteriovenous fistulas (AVFs) of the spinal cord are rare vascular malformations. This type was first described by Djindjian et al (1), classified as type IV spinal vascular malformation by Heros et al (2), and then categorized into 3 subtypes on the basis of the diameter, the magnitude of flow, and the number of feeding and draining vessels by Merland et al (3). This subdivision is important, because treatment options vary among the 3 hemodynamic subtypes. Surgery is currently accepted as the therapeutic technique in cases of type I (smallest) perimedullary spinal AVF (4). We reviewed the neuroradiologic images, treatment options, and clinical outcomes of 5 consecutive patients with type I perimedullary spinal AVF. The purpose of this report is to assess both the safety and efficacy of embolization in this type of lesion, highlight the steps of the embolization technique that increase success rate, and emphasize the important contribution of embolization to the treatment plan of these patients.
Patients and Methods
The clinical records and radiologic studies of 5 consecutive patients with type I perimedullary spinal AVFs who were treated at our institution were collected retrospectively. After clinical suspicion, the evidence of spinal vascular malformation was discovered first on MR imaging in all patients. The diagnosis of the type I perimedullary fistula and the distinction from other perimedullary fistula types (types II and III) were made by selective spinal angiography. The well-known distinction criteria have been described elsewhere (3, 5). Type I perimedullary AVFs are small-diameter, single fistulous communications, usually between the anterior spinal artery and moderately enlarged perimedullary veins. These lesions are usually located on the ventral surface of the conus medullaris or the filum terminale and are typically slow-flow lesions. Type II perimedullary fistulas are those AVFs intermediate in both size and flow velocity. These lesions generally have more than one feeder, usually including a dilated anterior spinal artery and perhaps including one or 2 posterior spinal arteries. There is more rapid shunt of blood through the fistula, with significant dilation of draining veins. Type III spinal perimedullary fistulas are giant AVFs with a multipediculated arterial feeder system. These lesions are typically high-flow and high-pressure shunts, with a markedly enlarged venous drainage system.
Clinical data are summarized in the Table. The patient group included 5 men ranging in age from 34 to 67 years (average, 51 years). The presenting symptoms were progressive paraparesis with sphincter disturbances in all patients. MR imaging in each case revealed flow voids over the surface of the lumbar and thoracal cord, representing enlarged vessels within the subarachnoid space. Signal intensity abnormality on T2-weighted images with or without cord swelling was the other invariable finding.
Summary of five patients with type I perimedullary spinal arteriovenous fistula
All the fistulas but one were located in the region of the conus medullaris; the last fistula was located in the filum terminale. In all but 2 patients, a single arterial supply to the perimedullary fistula originated from the anterior spinal artery. The arterial supply came from the posterolateral spinal artery in the second case, and the radiculopial artery in the last case (Table). Venous drainage was through the perimedullary veins in ascending fashion. An abrupt change in the caliber with or without sudden change in the direction of a blood vessel at the transition from feeding artery to draining vein is accepted as the fistulous point of AVF, which is considered as the optimal occlusion site.
Embolization procedures were performed under local anesthesia with sedation and systemic heparinization (5000–10000 U intravenously). The patients were treated by glue delivered through a 1.5F flow-directed microcatheter (Spinnaker Elite; Boston Scientific/Target, Fremont, CA). Catheter advancement was supported with a 0.012-inch guidewire (Mizzen; Boston Scientific/Target) to accomplish reaching the fistula site. The microcatheter tip was shaped into a small curve, which improves catheter navigation in the tortuous vessels. Because we had slow-flow fistula with no sump effect on the flow-directed microcatheter, a main arterial pedicle arising from the segmental artery (intercostal or iliolomber arteries) was catheterized by using microguidewire manipulation. Once the microcatheter was in the main pedicle (anterior or posterolateral spinal arteries), the microguidewire was withdrawn into the microcatheter, and then never extended beyond the microcatheter tip. Sharp turns or tortuosity of the feeder vessel were transversed by advancing the microcatheter and microguidewire as a unit. The details of this technique have been reported by Aletich et al (6). The microcatheter was inserted through the 5F guiding catheter, which was placed securely in the segmental artery. To improve proximal stiffness and enhance pushability of the microcatheter, the guiding catheter was placed within a 7F vascular long sheath.
Glue was injected only after the tip of the microcatheter was placed in the fistula site (Figs 1 and 2). On one occasion, the feeder was occluded proximal to the fistula site (Fig 3). This decision was based on the good understanding of the functional vascular anatomy of the spinal cord. After embolization, control selective spinal angiography was obtained in all cases. When a fistula could not be embolized, the malformation was exposed surgically via laminectomies according to the angiographic findings. The feeding arteries were visualized with standard microsurgical techniques and coagulated at the fistula site.
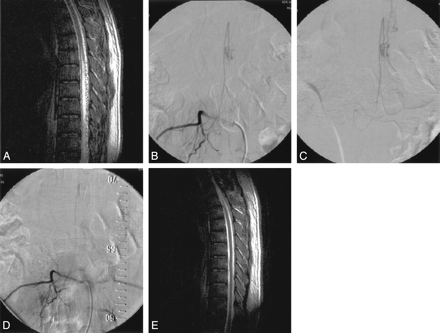
Case 2.
A, Sagittal T2-weighted MR image shows serpentine flow voids along the medulla spinalis with abnormal intramedullary signal intensity.
B, Selective angiogram of right L2 artery shows filling of the posterior spinal artery supplying the fistula at the level of conus medullaris.
C, Injection through the microcatheter (midway to fistula) depicts more clearly the fistula site located just distal to sharp bend, an ideal position for embolization. Please note that diameter of the feeder is slightly larger than the 1.5F (0.5 mm) microcatheter.
D, After embolization, selective right L2 artery angiogram reveals occlusion of the fistula with preservation of the posterior spinal artery.
E, Sagittal T2-weighted MR image 20 months after treatment shows that the signal intensity voids around the cord consistent with enlarged perimedullary veins and intramedullary abnormal signal intensity have disappeared.
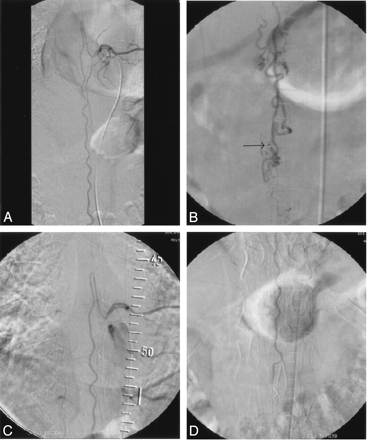
Case 1.
A, Selective angiogram of left T8 intercostal artery shows slightly enlarged artery of Adamkiewicz and tortuous anterior spinal artery.
B, Injection through the microcatheter placed just inside the fistula site at the level of conus medullaris shows typical dilated perimedullary veins. Arrow indicates microcatheter tip.
C and D, After embolization, selective angiograms of left T8 intercostal artery (C) upper and (D) lower part. The fistula is occluded with patent anterior spinal artery up to the level of conus medullaris.
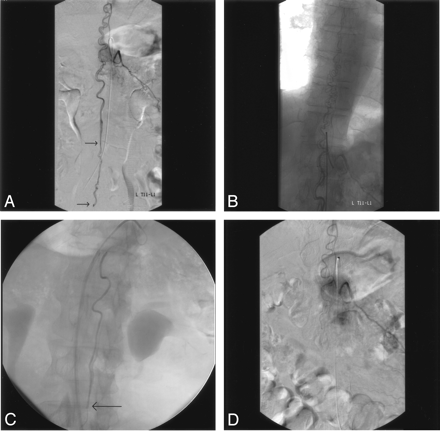
Case 4.
A and B, Selective angiograms of left T12 intercostal artery, early (A) and late (B) phases, show filling of the anterior spinal artery supplying the fistula located in the filum terminale. Arrows indicate 2 fistula sites. Note the serpentine perimedullary veins filling in ascending fashion.
C, Injection through the microcatheter placed at the level of conus medullaris shows first fistula (arrow) in the L3–L4 disk level. Please note triple-axial technique (guiding catheter within long vascular sheath) to improve pushability of the microcatheter. Despite the more caudal fistula located in the filum terminale, proximal occlusion of the feeder at the level of conus medullaris (current position) is safe.
D, After embolization, selective left T12 artery injection confirms occlusion of the fistula and preservation of the anterior spinal artery up to the level of conus medullaris.
Clinical follow-up was performed at 1 and 3 months and every year after treatment. Radiologic follow-up was performed at 3 and/or 6 months and 1 year after treatment with MR imaging. The fistula was considered to be cured when the previous neurologic status had improved and both abnormal perimedullary veins and pathologic spinal cord signals had disappeared on MR imaging.
The clinical status of the patients before and after treatment is presented in the Table. Lower extremity motor function was scored at 5 levels, whereas sphincter function was classified as good, fair, or poor.
Results
Four of the 5 patients were treated successfully by embolization, whereas one patient was considered unsuitable for embolization because of very thin arterial feeders. A single embolization procedure was performed and complete elimination of the fistula on postembolization angiography was achieved in all 4 patients. The last patient was operated on with uneventful postoperative period.
The follow-up period after treatment ranged from 30 to 49 months, with a mean of 41 months. All 5 patients improved clinically. Neurologic findings before treatment and at last clinical visit are summarized in the Table. During follow-up, all 5 patients had at least one MR imaging examination showing complete resolution of both abnormally dilated perimedullary veins around the spinal cord and intraspinal widespread increased signal intensity on T2-weighted images.
Discussion
Spinal perimedullary AVFs are intradural, extramedullary fistulas that are usually fed by the anterior spinal artery, but they may also be supplied by the posterior spinal arteries. These fistulas are further subdivided into 3 groups, on the basis of their hemodynamic pattern (3, 5). The main treatment protocol is to interrupt the shunt by surgery or embolization. During surgery, if the perimedullary fistula is located along the dorsal or dorsolateral aspect of the spinal cord, a standard posterior approach is used. The more common anteriorly located lesions require a rather difficult anterior or anterolateral approach, because it usually necessitates multilevel corpectomy requiring subsequent bone grafting and stabilization procedures. Lesions in the thoracic region are exposed via thoracotomies. Lower levels may require a retroperitoneal approach to achieve adequate exposure. Some anteriorly located lesions at conus medullaris may also be approached posteriorly (5, 7, 8).
Embolization is a viable alternative to surgery in patients with spinal perimedullay AVF, and may obviate complex surgical procedures. In general, the size of the fistula dictates the treatment in each case. The endovascular approach is proposed and usually the first line of treatment with surgery reserved for special circumstances in patients with large type III perimedullary fistula (5, 7, 9). The type II perimedullary fistulas having smaller arterial feeders with moderate size shunt are still suitable for embolization, and significant numbers of patients have been treated by using the endovascular approach (5, 7, 10, 11). The least frequent type, the type I perimedullary fistulas, by contrast, have very small feeders with low shunt. Embolization of these fistulas has neither been proposed as a first-line therapy (7, 4) nor established to be effective in a significant number of patients (5, 10, 11). In reviewing 3 large series dealing with all types of perimedullary fistula, among 14 type I fistulas that constitute 17% of all spinal perimedullary fistulas of these 3 series, only 2 (14% of all type I fistulas) underwent embolization (5, 10, 11). Because many of these lesions are not amenable to endovascular treatment, surgery has been a primary treatment option even in recent case reports (8, 12, 13). To date, there is no study dealing with the role of endovascular approach in this type of spinal fistula.
Although, embolization of spinal vascular lesions through the anterior spinal artery has been established as a feasible and effective method (14), the anterior or posterior spinal artery supply is still regarded as a dangerous feature that is considered a limiting factor for endovascular therapy. The embolization techniques have evolved in recent years as a result of advances in catheter and guidewire technologies, and the guidewire manipulation of the flow-directed microcatheter has been described recently by Aletich et al (6).
In considering treatment of the type I perimedullary fistula, the endovascular route has 2 obstacles: the diameter and the length of the feeder. The well-known over-the-wire catheters may never be suitable in this type of fistula, especially in patients with rather long feeding arteries, as in our 2 cases (1 and 4). As described by Aletich et al (6), in using this technique, we were able to navigate distal tortuous feeding vessels and selectively catheterize the fistula site in 4 of our 5 patients. Using the triple-axial technique (the guiding catheter inside the long vascular sheath) further increased our success rate by improving the pushability of the microcatheter.
In conclusion, at present type I perimedullary spinal fistulas have rarely been candidates for endovascular therapy. Use of the flow-directed microcatheter in conjunction with a proper guidewire, however, seems to have promising results in catheterizing safe points of these slow-flow fistulas. Once inside the fistula, embolization through this microcatheter with glue is very effective with consistent and encouraging results on long-term clinical and radiologic follow-up.
References
- Received February 20, 2005.
- Accepted after revision April 28, 2005.
- Copyright © American Society of Neuroradiology