Abstract
SUMMARY: A patient undergoing coil occlusion of a left internal carotid artery aneurysm was investigated by continuous arterial spin labeling MR imaging to evaluate perfusion territory mapping. Labeling was restricted to the left- or right-sided carotid artery by use of a separate neck coil. Before embolization, perfusion contrast was largely restricted to the labeled hemisphere. After embolization, perfusion contrast was created symmetrically in both hemispheres on labeling the right side, verifying sufficient collateral supply.
In carotid artery occlusive disease, the degree of stenosis is a critical parameter in the potential development of stroke.1 Knowledge of the amount of compromise of cerebral blood flow (CBF), of the affected perfusion territories, and of the status of collaterals would additionally be desirable for assessing the hemodynamic status of the brain and predicting the outcome. Perfusion MR imaging is increasingly being used for quantifying CBF and assessing cerebrovascular function. A totally noninvasive technique is arterial spin labeling, which exploits magnetically labeled arterial water as an endogenous, diffusible tracer to quantify perfusion from 2 sets of images obtained with labeling and a control condition. Approaches for mapping the flow territories of major brain-supplying arteries have also been suggested.2–6 For this study, we used continuous arterial spin labeling (CASL) with a local transmit coil at the neck for selectively labeling the blood of either the right or left carotid artery and a separate head coil for imaging.2 The goal was to evaluate the potential of perfusion territory imaging for an assessment of the hemodynamic status of the brain in a patient undergoing coil occlusion of the internal carotid artery (ICA).
Case Report
In a male 28-year-old patient, a dissecting aneurysm of the left ICA had developed after an accident during childhood and caused a minor stroke. Carotid balloon occlusion test revealed that the patient would tolerate permanent ICA occlusion because of sufficient collateral supply to the left hemisphere. Multiple Guglielmi detachable coils (Boston Scientific, Natick, Mass) were then inserted for the occlusion of the distal ICA aneurysm through a microcatheter. Postembolization angiograms verified total occlusion of the left ICA.
After obtaining informed written consent, CASL was performed on a 3T MedSpec 30/100 scanner (Bruker, Ettlingen, Germany), 1 day before and 3 months after embolization. A 6-cm-diameter surface coil was centered in the region of the right carotid bifurcation, and radiofrequency irradiation (1.2 W) and a magnetic field gradient (2.5 mT/m) along the z-direction were applied during labeling periods of τ = 3.5 seconds. Estimates of the labeling efficiency yielded α = 85%.7 One-hundred repetitions (TR, 7 seconds) were acquired with and without application of CASL during odd and even repetitions, respectively. To account for the finite transit time from the labeling plane to the region of interest, a postlabeling delay of w = 1.5 seconds was introduced before image acquisition. Eight axial sections (thickness, 5 mm; intersection distance, 6 mm) were recorded sequentially from superior to inferior with spin-echo echo-planar imaging (TE, 50 ms; bandwidth, 100 kHz; matrix, 64 × 64; FOV, 192 mm) and a quadrature helmet coil.8 T1-weighted images were recorded at the identical section positions as anatomic reference. In the pre-embolization examination, the whole procedure was repeated after repositioning the label coil for application of CASL on the left side.
All of the perfusion maps were registered to a 3D dataset acquired in a separate session for a direct comparison of individual sections. Images measured during the CASL and control conditions were averaged separately to create a difference map. Estimates of CBF were obtained from the signal intensity change, S̄CASL − S̄ctrl, according to the following9: 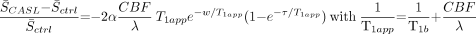 In this equation, a blood-brain partition coefficient λ is 0.9 mL/g, and a longitudinal relaxation time T1b is 1.3 seconds for cortical gray matter (GM). S̄CASL and S̄ctrl are averaged signal intensity amplitudes obtained with and without application of CASL, respectively, and T1app is the apparent longitudinal relaxation time.
In this equation, a blood-brain partition coefficient λ is 0.9 mL/g, and a longitudinal relaxation time T1b is 1.3 seconds for cortical gray matter (GM). S̄CASL and S̄ctrl are averaged signal intensity amplitudes obtained with and without application of CASL, respectively, and T1app is the apparent longitudinal relaxation time.
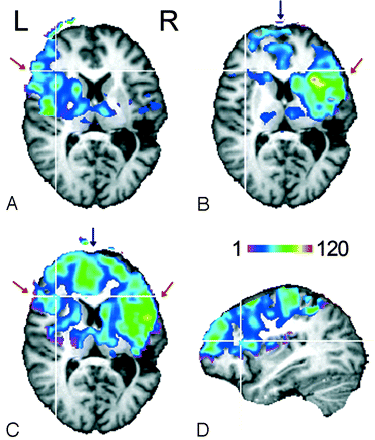
Quantitative perfusion maps obtained before left ICA occlusion are shown in Fig 1A, B. With left-sided CASL, perfusion-related signal intensity changes were found only in leptomeningeal branches of the left middle cerebral artery (MCA) flow territory (Fig 1B, red arrow), whereas with right-sided CASL, signal intensity changes were present in the flow territory of the right MCA (Fig 1A, red arrow) and also in the territories of leptomeningeal branches of both the right and left anterior cerebral artery (ACA) surrounding the interhemispheric fissure (Fig 1B, blue arrow). Figure 1C shows maps recorded with right-sided CASL after left ICA occlusion. Perfusion contrast was now created in both hemispheres in flow territories of the right and left ACA (Fig 1C, blue arrow), as well as that of the right and left MCA (Fig 1C, red arrow). CBF values averaged separately over the left and right hemisphere are summarized in the Table.
Perfusion territories of the (A) left and (B) right ICA recorded during the first examination and of the (C) right ICA recorded 3 months after coil occlusion of the left ICA. The color code indicates CBF values in milliliters per minute per 100 grams. Only voxels with a significant signal intensity change between label and no-label conditions (thresholded at P < .05) are displayed.
D, The coverage of the section package can be assessed from the sagittal section (left hemisphere).
Gray Matter CBF values of the ICA flow territories
Discussion
The pre-embolization results demonstrate that the flow territories of the left and right ICA can be delineated selectively with the 2-coil CASL technique. With the current setup, coverage in deeper brain structures (eg, the inferior parietal lobe) was limited by the sensitive volume of the helmet coil (length, 13 cm) and the angulation of the section package (Fig 1D). Improvement is straightforward by use of an appropriately modified imaging coil.
The distribution of perfusion contrast over both hemispheres after left ICA occlusion, which was observed on unilateral, right-sided CASL, verifies the existence of sufficient collateral supply, presumably via the circle of Willis as the primary pathway. This finding indicating a high sensitivity of the CASL technique agrees well with the result from the preceding carotid balloon occlusion test. Other than such qualitative correspondence, quantitative assessment of the adequacy of collaterals is obtained from the data in the table demonstrating equal postembolization levels of GM perfusion in both hemispheres within the experimental accuracy. The averaged CBF of GM in the unaffected right hemisphere and its variation by 10% between successive examinations are similar to values observed in a previous scan/rescan CASL study in normal volunteers.10 The data recorded before embolization in the flow territory of the left ICA seem to indicate a CBF reduction of borderline significance (P = .09 obtained from a section-by-section comparison and paired-samples t test). Alternatively, a prolonged transit time, which may be attributed to the presence of the aneurysm, could also lead to artificially reduced CBF values. Recently, δ = 1930 ± 110 ms was measured for the time the arterial water needs to travel from the labeling plane at the neck to the tissue in the ICA flow territory.11 A quantitative analysis within the framework of the 2-compartment model (compare equation 1 from Wang et al12) indicates that a transit time increase by 200 ms leads to a reduction of the signal intensity change by 13%, which would explain the observed interhemispheric difference.
Conclusion
Two-coil CASL has a potential to assess the significance of carotid artery stenosis and compensating collateral flow. Because of the possibility to perform repeated studies, it may be suited for screening purposes and for monitoring perfusion changes after therapeutic interventions, which is not adequately achieved by more invasive methods. A 10% range of normal variation and potentially confounding transit time effects impose limitations for detecting subtle CBF changes and should be addressed in further investigations.
Footnotes
H.E.M. and T.M. contributed equally to this work.
Paper previously presented at: Annual Scientific Meeting of the International Society for Magnetic Resonance in Medicine, May 6–12, 2006; Seattle, Wash.
References
- Received October 13, 2006.
- Accepted after revision November 27, 2006.
- Copyright © American Society of Neuroradiology