Abstract
BACKGROUND AND PURPOSE: A major concern during carotid artery stent placement is the potential for cerebral embolism. Diminishing the number of device manipulations across the lesion might reduce procedural stroke risk. For this purpose, we report our initial experience with carotid stent placement without the use of either balloon angioplasty or distal protection devices.
MATERIALS AND METHODS: Eighty-seven consecutive patients with 100 carotid stenoses compose this series. Ninety four of the 100 hundred stented carotid arteries were either symptomatic (58 [58%]) or had a greater than 70% stenosis (36 [36%]). Six percent of them were asymptomatic and had stenosis between 50% and 70%. Patients underwent neurologic evaluation before the procedure and during follow-up at 1, 3, 6, and 12 months and annually thereafter. Carotid sonography and plain films of the neck were performed immediately after the procedure and then at the same time intervals.
RESULTS: Primary stent placement was successful in 98 of 100 case subjects. In 2 case subjects, predilation was necessary before stent deployment. Neurologic periprocedural complications included 1 nondisabling and 1 disabling stroke and 5 transient ischemic attacks. The mean duration of follow-up was 23 months (range: 10–36 months). During the follow-up period, there were 5 deaths, all unrelated to the carotid disease, and no major stroke. The degree of stenosis decreased from a mean of 78.85% before the procedure to a mean of 21.23% immediately after.
CONCLUSIONS: In this series, carotid stent placement without the use of either balloon angioplasty or distal protection devices was safe and effective with a low incidence of periprocedural complications.
Carotid artery stent placement (CAS) has emerged as an alternative revascularization technique for extracranial carotid stenotic disease.1–3 Nevertheless, one of the limitations of CAS is the potential for embolic stroke caused by plaque dislodgement of atheromatous material.4,5 To prevent it, a variety of cerebral protection devices (CPDs) have been developed in the last years. Preliminary results have shown that these devices can significantly reduce thromboembolic complication during CAS.4,5 However, concerns have been raised regarding these protection devices, because they add further manipulation, cost, and risk to the procedure.6,7 It has been suggested that reducing the number of endovascular maneuvers in the supra-aortic vasculature can decrease the risk of plaque material dislodgment.7,8 Based on this information, it could be rationalized that the placement of a self-expandable stent without balloon dilation before or after stent deployment could minimize the risk of embolization and stroke by reducing the number of devices crossing the stenosis; if so, the use of a CPD would not be necessary in all cases. We report our initial experience with 100 consecutive carotid stent placement procedures without the use of balloon angioplasty before or after stent deployment, therefore without the use of CPDs.
Materials and Methods
Between June 2002 and October 2004, 87 consecutive patients with 100 carotid stenoses underwent stent treatment. There were 71 men and 16 women, with a mean age of 71 years (range: 48–88 years).
The indications for treatment were symptomatic and asymptomatic patients with carotid stenosis more than 70% or between 50% and 70% with evidence of high-risk plaque morphology or microembolism detected by transcranial Doppler (TCD). Exclusion criteria included bleeding diathesis, total occlusion lesions, disabling stroke, cerebral vascular malformations, degenerative cerebral diseases, cerebral tumors, illness impeding informed consent, and life expectancy less than 2 years (Table 1).
Major eligibility criteria
Sixty-four patients (89%) had comorbidities that placed them on the North American Symptomatic Carotid Endarterectomy Trial (NASCET) high-risk cohort of patients for endarterectomy9 (Table 2). Forty-seven stenoses were located on the right internal carotid artery and 53 on the left internal carotid artery. Thirteen patients had bilateral stenosis.
Characteristics of patients assigned to treatment
Fifty-eight lesions (58%) were symptomatic: nondisabling stroke (n = 34), transient ischemic attack (n = 18), and syncope (n = 6). Forty-two stenoses (42%) were asymptomatic: 36 stenoses (85.37%) were more than 70% and 6 (14.63%) were between 50% and 70%.
Preprocedural carotid ultrasonography was technically feasible in 85 case subjects. Based on recent literature, the plaque morphology was classified into 4 grades.10–12 Grades 1 and 2 were considered as high-risk plaque morphology; grade 3 was medium risk and grade 4 was low risk of stroke. All of the patients with a high-risk plaque were treated if stenosis was more than 50%.
Angiography (Integris; Philips Medical Systems, Best, the Netherlands) was performed before the endovascular intervention in all of the case subjects. The degree of stenosis before stent placement was quantified using the NASCET9 criteria and ranged from 50% to 99%, with a mean of 78.85%.
Before treatment, a baseline cerebral CT or MR imaging was performed in all of the patients but 6. All of the patients were also evaluated with power TCD. Intraprocedural TCD was performed in 15 patients. All of the patients underwent a neurologic examination by a neurologist before and 24 hours after the procedure.
Patients received oral aspirin (100 mg) and clopidogrel (75 mg) for at least 5 days before the procedure or, alternatively, a loading dose of 300 mg of clopidogrel at least 4 hours before carotid stent placement. Stent placement was performed under local anesthesia in all of the patients. At the beginning of the procedure, they received an IV bolus injection of heparin (100 U/kg).
A femoral approach was used in all but 4 of the cases (1 patient with bilateral lesions) who had either an extremely tortuous aortic arch (n = 2) or Leriche syndrome (n = 2). In these 4 procedures, direct carotid puncture of the ipsilateral common carotid artery followed by the placement of a 6F sheath was performed.
In the remaining 96 patients, in which the access was the femoral artery, a 6F sidewinder catheter was used to selectively catheterize the common carotid artery. A long exchange 0.035-inch stiff guidewire (Terumo, Somerest, NJ) was advanced into the external carotid artery and the 6F selective catheter was then exchanged for an 8F guiding catheter mounted coaxially over a 6F multipurpose catheter. Using “road-mapping,” the stenotic lesion was crossed by using a 0.014-inch guidewire. This was followed by advancement and deployment of a tapered stent (Acculink; Guidant, Santa Clara, Calif) of appropriate dimensions across the stenosis. In 2 patients in which the stenosis (99% tight stenosis) could not be passed with the stent delivery system, predilation with a 2.5-mm angioplasty balloon was necessary before stent deployment.
A cerebral angiogram was obtained at the end of the procedure. Hemostasis of the puncture site was achieved with a percutaneous closure device (Angio-Seal; St Jude Medical, Minnetonka, Minn). Once the procedure was completed, a plain radiograph of the neck was taken in different oblique projections to document the dimensions of the deployed stent.
In the first 58 cases, the patients were sent to the intensive care unit for their postoperative care. Because of the absence of adverse events in this group of patients, it was decided that the remaining 42 patients no longer needed to be closely monitored, and they were sent to a normal patient ward.
Postoperative medications included daily doses of aspirin (100 mg), clopidogrel (75 mg), and simvastatin (20 mg). Low molecular weight heparin (0.4–0.6 mg) was given twice, immediately after the procedure and 24 hours later.
Events were classified as fatal if death occurred as a direct result of stroke at any time after the procedure, disabling if the survivor had a persistent neurologic deficit causing disability of functional significance for more than 30 days after the onset of the symptoms (modified Rankin score ≥4), and nondisabling if symptoms lasted more than 7 days but resolved within 30 days with no disability of functional significance (modified Rankin score ≤3). A transient ischemic attack was defined as a focal retinal or hemispheric event from which the patient made a complete recovery within 24 hours.
Follow-up assessment was carried out with a CT or MR imaging examination 48 hours after the stent placement. On discharge, the patients were followed up clinically with Doppler sonography and with plain films in different angle positions of the neck at 1, 3, 6, and 12 months and annually thereafter. The data were analyzed according to a Student t test for paired samples and Fisher exact test for small samples when required.
Results
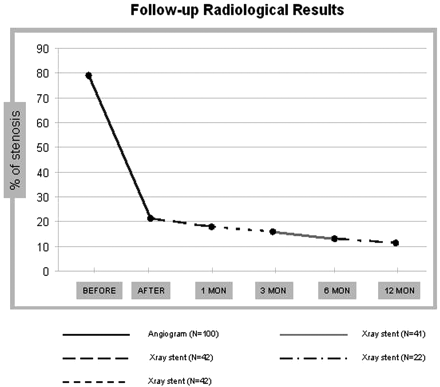
Primary stent placement was successful in 98 of 100 patients. In 2 patients, predilation was necessary before stent deployment. In most patients, the immediate angiography showed significant improvement in the degree of stenosis (Fig 1). On average, the degree of stenosis decreased from a mean of 78.85% before the procedure to a mean of 21.23% immediately after stent placement. In some patients, stents expanded instantaneously and almost completely (Fig 2); however, most of them improved gradually over time (Fig 3). On the plain film of the neck obtained during the follow-up, the average degree of stenosis continued to decrease (the severity of residual stenosis impinging on the stent was measured using the NASCET9 formula, assuming that the distal caliber of the stent represented the true diameter of the distal internal carotid artery, from 21.23% immediately after stent placement to 17.73% at 1 month, 15.95% at 3 months, 13.04% at 6 months, and 11.35% at 12 months (Fig 4). The average diameter of residual stenoses immediately after the procedure was significantly smaller than the average of stenoses on preprocedure angiograms (P = .002), as well as the difference in the degree of stenosis by x-ray obtained between 1 month and 3 months of follow-up (P = .03). The differences between 3 and 6 and between 6 and 12 months, however, were not statistically significant (P > .05).
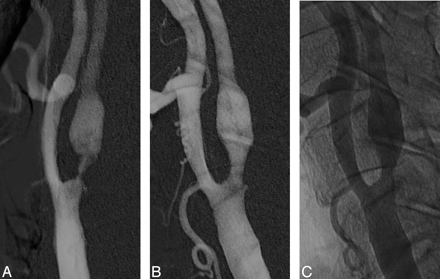
A, Left common carotid artery digital subtraction angiogram, showing a severe stenosis of the proximal internal carotid artery, measuring more than 90%.
B, Immediate poststenting control with residual stenosis less than 30%.
C, Nonsubtraction image of the immediate poststenting angiogram.
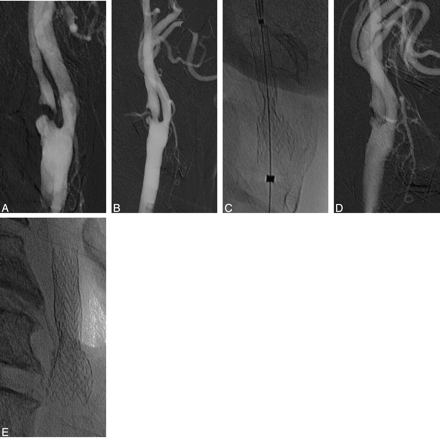
A, Right CCA DSA, showing a subocclusive stenosis.
B, Immediate poststenting control with residual stenosis less than 50%.
C, Plain film immediately after stent deployment.
D, Final control angiogram with complete expansion of the stent.
E, Plain film at the end of the procedure showing total expansion of the stent.
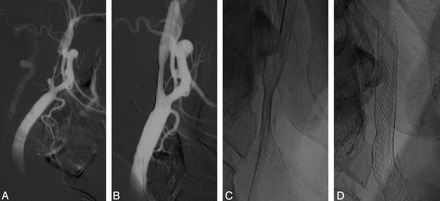
A, Left CCA DSA, lateral view, showing a long thigh stenosis of the proximal ICA.
B, Repeat DSA, lateral view, immediately poststenting.
C, Plain film showing residual stenosis <50%.
D, Plain film at the first-month follow-up, showing a complete expansion of the stent.
Follow-up radiologic results demonstrating the variation in stenotic lumen in the preprocedure and postprocedure angiogram and subsequently in plain films of the stented artery at 1, 3, 6, and 12 months.
A follow-up CT or MR imaging was performed in 81 patients. These studies showed no changes compared with the prestenting CT or MR images. During the follow-up intrastent restenosis, >70% was detected by Doppler sonography at 3–6 months after the stent placement procedure on 5 otherwise asymptomatic patients. Intrastent angioplasty by using cerebral protection (Accunet; Guidant) was performed in these patients. At the latest sonography follow-up, the lumen of these stents has remained patent with no signs of restenosis.
The periprocedural neurologic complications included the following (Table 3): transient ischemic attack (n = 5), motor dysphasia (n = 1), and left brachial hemiparesis (n = 1). During the initial 30-day follow-up, neurologic complications remained in 2 patients (2%). Partial recovery within 72 hours was observed in the patient with motor aphasia, and partial disability was present in the other patient at the 9-month follow-up. During the mean follow-up period (23 months; range: 10–36 months), there were 5 nonrelated procedure deaths (postcardiac surgery) and no fatal stroke. The causes of death included myocardial infarction (n = 2), ventricular arrhythmia (n = 1), massive pulmonary embolization (n = 1), and multiple organ failure (n = 1), which occurred from 35 days to 9 months after stent placement.
Complications in the periprocedural and mean follow-up period
With regard to the neurologic complication rates found between the high-risk and the low-risk plaque morphology group, a similar neurologic complication rate was observed in both groups. Four of the patients developed hyperperfusion syndrome without any abnormal findings on the CT or MR imaging. Other nonneurologic complications included a neck and inguinal hematoma after direct carotid and femoral puncture, respectively.
Discussion
CAS has proved to be as effective as carotid endarterectomy (CEA) in alleviating carotid stenosis in high-risk patients.13 However, the potential for cerebral embolism during the procedure generates a great concern regarding the safety of CAS. The American Heart Association Consensus Conference Recommendation has set the maximum acceptable complication rate of stroke after carotid intervention as 6% for patients with symptomatic lesions and 3% for asymptomatic patients.14–16 Any alternative technique with a complication rate exceeding these recommendations is considered unacceptable. Clinical experience with CAS has shown relatively high 30-day stroke/death rates ranging from 3.98% to 10%.1,17–19 Because of this relatively high incidence of adverse effects, efforts have been made to reduce their incidence by using CPDs during CAS.4,5,20,21 An extensive literature review indicates that the use of CPD can decrease the incidence of 30-day combined stroke and death rate after CAS from 5.5% without the use of CPD to 1.8% with CPD.4 Wholey et al,22 on the global carotid artery stent registry, reported a reduction in stroke rate and procedure-related death from 5.29% in patients without protection to a 2.23% rate when using protection. The recent Stenting and Angioplasty in Patients at High Risk for Endarterectomy (SAPPHIRE) Study also demonstrated that stroke rates are similar between CAS with cerebral protection and CEA in high-risk patient populations.13 Although these reports are encouraging, there is evidence that neuroprotected CAS is associated with predominantly silent cerebral ischemia in approximately 25% of the patients.21 Other reports have pointed out that the use of CPDs does not offer a full guarantee of completely eliminating the risk of embolic complications during CAS.1,22–24 Furthermore, CPD itself can cause severe spasm and dissection with subsequent minor stroke.25,26
Other potential drawbacks of CPDs include the passage of a CPD through the stenotic lesion that entails a potential risk of dislodgement of unstable plaques, the intolerance to balloon occlusion devices in many patients who have contralateral carotid disease or absence of intracranial communicant arteries, failed deployment of the CPD or difficulty in its retrieval because of complicated anatomy, and embolus that can potentially escape during removal of the CPD. These disadvantages have led some authors to be skeptical about the value of protection devices.21,24
The primary objective of this study was to evaluate the safety and efficacy of nonprotected CAS without the use of balloon dilation before and after stent deployment. The rationale behind this objective was based on the observations of several authors.20,27 Al-Mubarak et al20 demonstrated by TCD that the embolic events are related to the endovascular manipulations. Men et al27 stated that less catheter and guidewire manipulations and the elimination of balloon dilation before and after stent deployment should result in a decreased number of Doppler-detected microembolic signals (MES) during the procedure. MES are detected by TCD during all of the procedural phases of CAS: sheath placement, guidewire manipulation, CPD introduction and placement, balloon predilation and postdilation, and CPD removal.20,28 In our study, more MESs were recorded by TCD during advancement of a wire across the stenosis than during the stent delivery system advancement and deployment across the stenotic segment. Although TCD has proved that the procedure-related embolization during CAS is higher than that during CAE, the clinical significance of microemboli, however, is not clear.28
The incidence of perioperative major stroke in our series was 1%, with 1 minor stroke. No fatal major strokes or deaths were related to the procedure. The low incidence of major strokes (1%) compares favorably with the 6.7% overall rate of perioperative death and stroke and the 2% rate of permanently disabling stroke and death reported on the NASCET Trial,9 with the overall 30-day stroke and death rate of 7.4% reported by Roubin et al,17 with the CAS 6.2% stroke rate of the SAPPHIRE Trial,13 and with the 1.8% and 5.5% rate for those treated with and without CPD, respectively, reported by Kastrup et al.4
With the exception of 2 patients in whom we could not pass the stent across the stenosis and we had to predilate with a small balloon, the stent passed without much difficulty across 98 lesions and spontaneously expanded in all of them, including those that were near occlusive. This could be related to the fact that new generations of self-expanding stents have become smoother with lower profiles, allowing a less traumatic passage through stenotic lesions.
Several reports have stressed the fact that high-risk morphology plaques have a high propensity to embolize and cause stroke.10,29,30 Although there is no strong evidence at present that supports the selection of patients for treatment based on sonography examination, it has been suggested that measurement of echolucency together with degree of stenosis may improve the selection of patients for surgical treatment.11 This is the reason to include asymptomatic patients presenting with stenoses between 50% and 70% associated with high-risk morphology plaques in our series. High-risk morphology plaques were found in 8 of the asymptomatic and in 21 of the symptomatic patients. Periprocedural neurologic complications were observed in 6.8% (n = 2) from a total of 29 patients with high-risk plaque morphology and in 7.1% (n = 5) from the low-risk plaque morphology group. Although the neurologic complication rates were similar in both groups, the severity was significantly higher in the high-risk group patients, because the 2 complications from this group represent 1 minor and 1 major stroke, whereas the 5 complications seen in the low-risk group are all transient ischemic attacks. The higher periprocedural risk of stroke (P = .02) found in our study on patients with a high-risk plaque morphology is in agreement with findings in previous reports.9,10,26
Although carotid stent placement without the use of balloon dilation and CPD has been sporadically described in case reports,8,24 little information on its efficacy and safety is available from clinical investigations with a sizable patient sample, except for the report by Lownie et al8 of 21 patients treated without balloon dilation. From our experience with 100 stenotic lesions in 87 patients treated with stent placement, we found that primary stent placement without balloon dilation is technically feasible in most patients (98%) with carotid stenosis. The procedure proves to be simple and time saving without significant technical difficulty. The 1% of 30-day major neurologic events compares favorably with the rates reported with CEA and conventional CAS. The 5% overall death rate reflects the high-risk patient population involved in the study. None of the casualties in our study occurred in the 30 days after the procedure, nor were they neurologically related. The notable reduction in major neurologic events after the stent placement procedure may reflect the advantages of a simplified manipulation and the shorter procedure time. The maximum stent expansion occurred during the procedure (stenosis decrease from 78.85% to 21. 23%; P = .002), followed by a gradual further expansion over the next 12 months. Despite the initial incomplete expansion of the stent, during the mean 23-month follow-up, only 5 patients developed restenosis caused by intrastent myointimal hyperplasia. All of these patients were asymptomatic and were detected during the Doppler sonography control.
They were all successfully treated with intrastent angioplasty. Stent placement without balloon dilation allows that disruption of the atheromatous plaque is not caused acutely, so dislodgement of atheromatous material is less likely to occur.
Gradual expansion of a self-expanding stent could also offer the additional benefit of potentially reducing the risk of reperfusion hemorrhage after restoration of carotid flow. In this series, we observed in many patients a continuous expansion of the self-expanding stent over the follow-up period. Although hyperperfusion did occur in 4 of the patients, all of them had a TCD pattern of mild hyperdynamic cerebral blood flow during the first 24–48 hours after stent placement. It is our belief, and this is supported by others authors,8,24 that there is no need to fully expand the deployed stent, because the neurologic events generally are caused by artery-to-artery embolus and not by hemodynamic effects. A separate complication described in the CAS literature is vagal reaction because of balloon dilation at the level of the carotid bulb. This complication is eliminated if neither prestenting nor poststenting balloon dilation is used.
Potential limitations for the use of this technique include those patients with extremely tight stenosis in which a stent delivery system may fail to cross without predilation, as occurred twice in this series; and in calcified stenosis in which the radial expanding force of a self-expanding stent might fail to overcome the resistance presented by a rigid calcified arterial wall without the aid of poststenting balloon dilation, though this was not seen on any of the 100 cases of stent placement in this series. If the stent failed to expand during the follow-up, a CPD could then easily cross a lesion and protect the brain during balloon dilation of the stent, as occurred in 5 of our patients between 3 and 6 months after the procedure.
Conclusions
In this series, CAS without use of either balloon angioplasty or distal protection devices was safe and effective with a low incidence of periprocedural complications. The results from this series provide grounds for further clinical investigations, especially for a prospective, randomized study comparing this method of CAS without balloon dilation and protection devices against CAS using CPD with prestent and poststent deployment balloon dilation.
References
- Received August 21, 2006.
- Accepted after revision December 12, 2006.
- Copyright © American Society of Neuroradiology