Abstract
SUMMARY: Osteoporosis is a common cause of vertebral compression fractures. Although vertebroplasty is used to treat the pain, the risk of additional compression fractures is very high in these patients. Adequate evaluation and management of the underlying osteoporosis is critical to reducing the risk of subsequent fractures. Such an evaluation involves understanding the underlying physiology of osteoporosis and the role of calcium, vitamin D, prescription medication, and lifestyle changes. This brief review is intended to familiarize neuroradiologists with these aspects so they can advise patients about optimizing fracture risk reduction.
With the widespread adoption of percutaneous vertebroplasty for treatment of painful osteoporotic fractures, radiologists find themselves directly involved in the care of elderly patients with complex medical problems. Simply placing cement into painful fractures without regard for optimizing the medical therapy of these patients may compromise long-term care. Multiple studies have documented that osteoporosis evaluation and treatment is performed on only a small proportion of patients after an osteoporotic fracture.1
Numerous barriers to optimizing the proportion of patients undergoing appropriate osteoporosis evaluation and receiving treatment have been identified, but no easy solutions have been found. Increasing pressure is being placed on specialists who treat fractures, such as orthopedic surgeons and interventional radiologists, to play a more active role in the overall care of patients with osteoporosis. Indeed, patients treated with vertebroplasty may have multiple sequential fractures with resultant vertebroplasty procedures, placing the radiologist in the role of long-term caretaker of at-risk patients. Traditionally, radiologists have not been involved in this aspect of the care of patients with fractures, so they may lack the appropriate knowledge, training, and experience about osteoporosis evaluation and treatment.
The purpose of this review is to familiarize practicing radiologists with the current understanding of the mechanisms behind poor bone health and to provide a context for current medical treatment strategies. Further, details about the most relevant available medical treatments will be offered. Last, suggestions will be made regarding when and to whom subspecialty referrals might be made. It is not intended for this review to necessarily render the radiologist an expert in evaluation and management of the patient with osteoporosis. Rather, we hope that the information presented will encourage radiologists to look beyond the vertebroplasty procedure and assess whether a patient with osteoporosis is receiving optimal medical treatment, with the goal of improving the overall health of patients undergoing vertebroplasty.
High Risk of Subsequent Fracture
Vertebral fractures account for almost half of all symptomatic osteoporotic fractures and have clinical implications beyond pain. Increased mortality is associated with a vertebral fracture but becomes evident well beyond 1 year following vertebral fracture onset, in contrast to the immediate increased mortality after a hip fracture, which then decreases gradually with time.2 The risk of a subsequent vertebral deformity is seven- to tenfold higher in those individuals who have experienced a previous vertebral deformity,3and the incidence of new vertebral fracture within a year of a vertebral fracture approaches 20%.4 Quality-of-life scores decrease with increasing numbers of vertebral fractures.5 Data from Europe indicate that prevalent vertebral deformity predicts incident hip fracture with a rate ratio of 2.8–4.5, which increases with the number of vertebral deformities.6 Therefore, any patient presenting with a painful fracture for consideration of vertebroplasty must also be considered for systemic therapies aimed at decreasing future fracture risk, including fractures of the hip, wrist, and spine.
Bone Remodeling and Fracture Risk
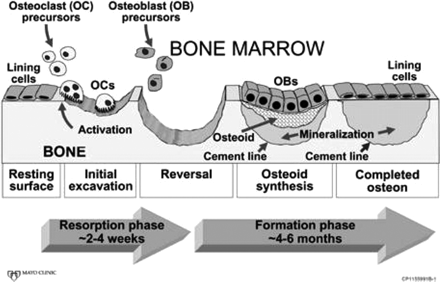
Bone is a dynamic tissue that is constantly in a process of turnover or self-renewal, in response to mechanical stress and hormonal changes and to maintain mineral homeostasis. This process, called “bone remodeling,” occurs at discrete bone surfaces and involves the integrated and sequential actions of osteoclasts and osteoblasts (Fig 1). Tight coupling of bone resorption by osteoclasts and bone formation by osteoblasts are required to maintain the integrity of the skeleton. Many disorders of skeletal fragility are the consequence of alterations in this balance.
Bone remodeling sequence. A cartoon depiction of the sequential action of osteoclasts and osteoblasts to remove old bone and replace it with new bone. For simplicity of illustration, the cartoon shows remodeling in only 2 dimensions, whereas in vivo, it occurs in 3 dimensions, with osteoclasts continuing to enlarge the cavity at one end and osteoblasts beginning to fill it in at the other end. Reproduced with permission of the American Society for Bone and Mineral Research from J Bone Miner Res (2005;20:177–84).
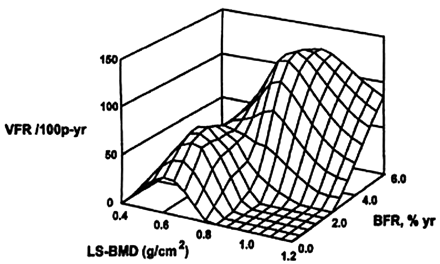
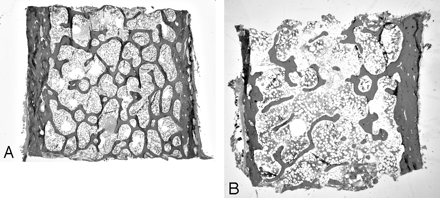
Each bone-remodeling cycle leads to a net loss of bone due to a modest deficiency in the amount of new bone formed relative to old bone removed.7,8 Acceleration of the bone remodeling rate, which is common in many metabolic bone diseases including postmenopausal osteoporosis, thereby leads to a net loss of bone.7 Elevated bone remodeling also produces a deterioration of bone microstructure, with loss of trabecular connectivity (Fig 2). In addition, new bone is less mineralized than existing bone and thereby less “strong” than more mature and fully mineralized bone. These changes in microstructure and material properties of bone tissue result in lowered mechanical strength, which, in addition to the absolute loss of bone mass from the high remodeling rate, increases the risk of fracture. Postmenopausal women with osteoporosis and high rates of remodeling have a higher risk of fracture than those with similar bone mineral density but low remodeling (Fig 3).9 Lowering of bone remodeling has therefore become a major therapeutic strategy for preventing bone loss, and numerous studies have validated that drugs with this effect reduce the risk of fracture.10
High bone remodeling is associated with increased risk of fracture in postmenopausal women. The x-axis shows lumbar spine bone mineral density (LS-BMD), the y-axis shows vertebral fracture rate (VFR), and the z-axis shows bone remodeling rate (BFR) from iliac crest bone biopsies. The peaks of fracture rate occur with low bone density and a high remodeling rate. 100p-yr indicates 100 patients per year. Reprinted with permission from Elsevier from Riggs et al.9
Microstructure of normal (A) and osteoporotic (B) bone. Iliac crest bone biopsy shows normal trabecular connectivity (A) and loss of connectivity in osteoporotic bone (B). The reduction in total bone mass is also evident in osteoporotic bone (original magnification ×1.25).
Assessment after a Low-Trauma Fracture
The primary goals in evaluating an individual after a low-trauma fracture are to determine the bone mass and to exclude secondary causes of bone loss. For those who already have been diagnosed with osteoporosis and are on appropriate therapy, the goal is to assess the adequacy of that therapy. Bone mineral density measurement by dual-energy x-ray absorptiometry (DXA) of the hip and spine is useful in patients not on prescription medications to assess bone mass and in treated patients to assess the adequacy of current therapy. Although other methods of assessing bone mass (quantitative CT, heel sonography, peripheral DXA) exist, central DXA remains the gold standard for longitudinal measurement and for defining osteoporosis according to the WHO criteria in patients who have not had a low-trauma fracture.11 Patients who have sustained a low-trauma fracture and have osteopenia by DXA meet the diagnostic criteria for osteoporosis. A DXA bone mineral density is a static measurement of bone mass at 1 point in time and cannot determine bone loss without a previous measurement for comparison. Although the rate of remodeling (and presumed rate of bone loss) can be assessed with blood and urine tests, the routine use of these in clinical practice remains controversial. A recent fracture will elevate these biochemical markers of bone turnover for some time (at least several months)12; therefore, measurement is less useful in the setting of a recent fracture.
Individuals with low bone mass and a low-trauma fracture have osteoporosis and will benefit from a multistep intervention to lower the risk of subsequent fractures. Epidemiologic studies have identified a number of risk factors for osteoporosis and fracture that can be determined by medical history and examination (Table 1).13 Similarly, most secondary causes of osteoporosis can be identified by history and examination (Table 2). There are no universal guidelines for the most cost-effective approach to excluding secondary causes of bone loss in patients without an obvious etiology after history and examination. One small study of 173 postmenopausal women, who, after history and examination, did not have an identified cause of bone loss, found that a simplified laboratory testing strategy of measuring serum calcium, 25-hydroxyvitamin D, and 24-hour urinary calcium for all women and thyroid stimulating hormone for women on thyroid hormone replacement would identify 92% of patients with secondary osteoporosis.14 This assumes that basic blood tests of kidney function, liver function, and blood count have been performed recently. Men and premenopausal women represent special groups that benefit from referral to a specialist for additional evaluation.
Risk factors for osteoporosis and fracture
Disease contributing to secondary osteoporosis
Treatment of a Patient with Osteoporosis
Nonpharmacologic Interventions
Multiple nonpharmacologic interventions, including diet, smoking cessation, exercise, and implementation of proper biomechanics during lifting, are potentially important in patients presenting for vertebroplasty. These aspects are discussed below.
Smoking Cessation and Avoidance of Excessive Alcohol Intake.
Smoking and excessive alcohol consumption are unhealthy habits associated with an increase in the risk of fractures in epidemiologic studies.15 Changing these habits benefits many organ systems including bone. Referral to appropriate resources for aid in tobacco cessation and dealing with excessive alcohol intake may be necessary for some patients.
Adequate Intake of Calcium and Vitamin D.
Calcium and vitamin D intake alone may not prevent all bone loss that occurs with postmenopausal estrogen deficiency or other conditions associated with bone loss. Although inadequate calcium and vitamin D intake has been associated with an increase in the risk of fractures and may contribute to the development of osteoporosis, the antifracture efficacy of calcium and vitamin D supplementation is at best very modest.16–18 Even so, the National Academy of Sciences recommends that adults older than 50 years consume 1200 mg of calcium daily.
The first step in counseling patients regarding calcium supplementation is to estimate how much calcium is obtained from current dietary sources and to recommend that the patient make changes to increase that intake, if possible. In general, dairy products and calcium-fortified foods (such as some orange juices) are sources of high amounts of calcium. Food frequency questionnaires are simple and easily administered if needed.19 If even after dietary changes, calcium intake remains below ideal, supplements can be taken. Because of limits to intestinal absorption, no more than 500–600 mg of calcium supplements should be consumed at one time. Calcium citrate preparations are easily absorbed, even in a low-acid environment (individuals who use gastric acid–lowering drugs), but will have less calcium per pill than calcium carbonate. Calcium carbonate taken with food is generally well-absorbed, less expensive, and available in tablet and chewable forms in many flavors.
The National Osteoporosis Foundation currently recommends vitamin D intake of 800–1000 U daily for patients with osteoporosis, which is higher than past recommendations. The increase in the recommended amount of vitamin D reflects recent studies demonstrating that higher vitamin D levels suppress bone remodeling further. However, there are no trials with fracture as an end point that have clearly defined the “optimal” vitamin D level. Vitamin D also has important effects on muscle function and strength, and there is growing evidence that optimizing vitamin D levels may reduce the risk of falls.20 This reduction is a major goal for osteoporosis management. Although vitamin D is made in the skin by ultraviolet radiation, aging skin, sun block use, and residence in a northern latitude often limit the amount produced by sunlight exposure. Milk is fortified with vitamin D, but most other dairy products are not, and few other foods naturally contain it or are fortified with it. Over-the-counter preparations are readily available, often combined with calcium. There is much debate about whether vitamin D3 (cholecalciferol) is superior to vitamin D2 (ergocalciferol). A recent study suggests that there is no difference in these preparations for obtaining adequate vitamin D levels.21 Toxicity from excessive vitamin D is rare.
Participation in Weight-Bearing Activity.
Bones are designed to bear weight. Although the exact mechanism of the mechanosensing is not known, it is clear that weight-bearing activity decreases bone remodeling. Although one would like a randomized controlled trial demonstrating that physical activity prevents fractures, methodologic issues of power, compliance, blinding, drop-outs, and long-term follow-up make such a study virtually impossible. Evidence does exist from prospective observational cohort studies that physical activity reduces the risk of fragility fractures in both men22 and women.23 Benefits were observed with moderate levels of physical activity, including walking, which are readily attained by engaging in recreational sports and other forms of exercise. The benefit in fracture risk seen with physical activity may be related to improvements in bone mineral density, decrease in the risk of falls, or a combination of these 2. Initiating physical activity even at an elderly age can be beneficial to reduce the risk of falls.24
Avoidance of Falls and Optimization of Biomechanics.
Preventing falls is of paramount importance for the patient with osteoporosis. A careful assessment of the home environment may reduce the fall risk by recommending removal of loose scatter rugs and installation of grab bars in the bathroom. Use of a cane or walker for gait instability is also important.
Most vertebral fractures occur during routine daily activities, either from a fall, a bending/twisting motion of the torso, or lifting a load that is too heavy for the weakened vertebrae to bear. Patients having pain from a recent vertebral fracture limit their activity because of the pain. However, as the pain improves, patients will become more active and need to be advised to avoid unsafe movements that excessively load the vertebrae. This is especially important in the postvertebroplasty population, not only because the procedure seems to offer outstanding pain relief but also because of ongoing concern about the apparent increased risk of new fracture following vertebroplasty.25 Advising patients to reduce the weight they lift by carrying smaller loads and forgoing certain activities such as lifting grandchildren and shoveling snow is also important. Patients may benefit from referral to a physiatrist or physical therapist for instruction in exercises aimed at maintaining proper alignment and good posture to limit progressive kyphosis from additional compression fractures.26 Patients often resist making changes to their activity or using a cane or walker because of the fear of admitting aging and frailty. Discussing these recommendations in the light of an overall strategy to maintain independence by limiting future fractures is sometimes helpful.
Pharmacologic Treatments
For patients with osteoporosis and particularly those who have fractured vertebrae, nonpharmacologic interventions are necessary but alone are not sufficient treatment to maximally reduce the risk of future fracture. The last decade has seen a dramatic increase in the number of drugs for the treatment of osteoporosis. Here, we will discuss only those medications that are currently US Food and Drug Administration (FDA)-approved for the treatment of osteoporosis (Table 3, reviewed in detail elsewhere10). Outside the United States, there are additional therapeutic options. The medications fall into 2 broad categories based on their effects on bone physiology. There are no head-to-head trials with fracture outcomes of any of these medications or of combinations of these medications that would support a claim of superiority.27
Approved pharmacologic agents for treatment of osteoporosis
Anticatabolic Therapies.
Drugs in the anticatabolic category primarily target the osteoclasts and lower the rate of bone remodeling. In general, they produce modest gains in bone mineral density but have more dramatic effects on bone remodeling, which together translate into a reduction in the risk of fractures.
Bisphosphonates.
The bisphosphonates are first-line drug therapies for osteoporosis treatment. They reduce bone resorption by inhibiting the activity of osteoclasts and shortening their life span. Because these drugs are incorporated into bone and remain there for years, they can be given intermittently. Oral bisphosphonates are poorly absorbed, so they must be taken first thing in the morning when the stomach is completely empty and only with plain water. Nothing else can be taken by mouth (ie, no other beverage, food, or medication) for 30 minutes to 1 hour, depending on the particular drug. Patients cannot lie down after taking the medication to reduce the risk of the medication refluxing into the esophagus and causing injury to the mucosa. All bisphosphonates approved by the FDA for treating osteoporosis in postmenopausal women have been demonstrated in randomized placebo-controlled trials to reduce the risk of fractures.28–31 Although all these agents have shown the ability to reduce vertebral fractures, effective hip fracture reduction has been demonstrated only for alendronate,28 risedronate,32 and zoledronic acid.29
Side effects from oral bisphosphonate preparations are most commonly gastrointestinal symptoms. The oral preparations are not used in individuals with esophageal motility disorders or active ulcer disease or who are not able to comply with dosing instructions. Very rarely, osteonecrosis of the jaw has been reported with all bisphosphonates, including those used to treat osteoporosis.33 The mechanisms of this are not understood and on-going investigations designed to better define the entity, its incidence, and potential treatments are in progress. This drawback has received wide coverage in the lay press. In general, the risk is so low compared with the risk of fracture that the risk/benefit profile favors the use of this class of drugs to lower fracture risk in patients with osteoporosis. All patients receiving bisphosphonates are advised to see their dentist regularly, practice good oral hygiene, and report any dental problems promptly.
Estrogen.
Numerous studies, including the Women's Health Initiative study, have demonstrated fracture-risk reduction with estrogen use in postmenopausal women.34 However, estrogen (alone or with progesterone) is no longer recommended as a first-line treatment for osteoporosis. It may be used for osteoporosis prevention in women experiencing menopausal symptoms, but the risk/benefit ratio and the availability of good alternatives make it rarely indicated for osteoporosis otherwise. The risks include thromboembolic events, stroke, breast cancer, and coronary heart disease.
Raloxifene.
The only selective estrogen-receptor modulator approved for use in the treatment of osteoporosis is raloxifene. Raloxifene binds to the estrogen receptor and mimics the activity of estrogen in some tissues while not activating and inhibiting estrogen receptors in other tissues. Hence, it has been demonstrated to prevent vertebral fractures35 but does not cause uterine bleeding36 and is comparable to tamoxifen in preventing breast cancer.37 The effects of raloxifene on bone are similar to those of estrogen but are probably a little weaker. Similar to estrogen, raloxifene has been associated with an increased risk of thromboembolic events. No cardiovascular benefit or harm has been found with raloxifene. Vasomotor symptoms (menopausal hot flashes) may increase. It is not recommended for use in patients with a history of estrogen-sensitive malignancy, most notably breast cancer.
Salmon Calcitonin.
Salmon calcitonin has actions on bone similar to endogenous human calcitonin: decrease in bone resorption by effects directly on osteoclasts. It is approved for treatment of osteoporosis on the basis of demonstrated ability to reduce the risk of vertebral compression fractures.38,39 The original formulation required daily subcutaneous injection, but current formulation as a once-daily nasal spray is vastly more acceptable to patients. Side effects of the nasal spray are largely only local nasal irritation. One unique property of salmon calcitonin compared with other osteoporotic therapies is that some studies suggest that it may have analgesic properties in the setting of pain from an acute compression fracture.40 This is not an approved use of this drug. Calcitonin is generally thought to be weaker than the other anticatabolic drugs. The route of administration and good tolerability make this drug suitable for patients who do not tolerate other therapies or have complicated medication regimens.
Anabolic Therapy.
By definition, anabolic drugs stimulate new bone formation, consequently producing larger increments in bone mineral density than anticatabolic drugs. This is accompanied by qualitative improvement in the trabecular microarchitecture and enhanced cortical thickness, which may be more beneficial in the long term. Yet, to our knowledge, no studies have been performed to show that this produces a greater reduction in fracture risk than anticatabolic therapy.
Teriparatide.
The only anabolic therapy approved for use in the United States is teriparatide. Teriparatide is a recombinant fragment (amino acids 1–34) of human parathyroid hormone. It has been shown to reduce the risk of vertebral and nonvertebral fractures in postmenopausal women with osteoporosis.41 Men with osteoporosis appear to respond similarly to women in terms of improvement in bone mineral density, but no fracture data are available.42 Currently, teriparatide is only available as a once-daily subcutaneous injection and is quite costly. Side effects include local irritation at the site of injection, bone pain, and dizziness. High doses of teriparatide given to laboratory rats resulted in an increased risk of osteosarcoma. No increased risk of osteosarcoma has been found in humans, but the drug is not generally given to patients with an increased risk of osteosarcoma (children and patients with a history of skeletal radiation therapy or Paget disease of bone). The general consensus is that teriparatide is useful in patients with severe osteoporosis (by bone mass density [BMD] or multiple fractures), relatively younger patients with very low BMD, and in some patients on long-term glucocorticoid therapy. This drug is generally not used in combination with anticatabolic drugs.43,44 The duration of treatment is currently limited to 2 years. After treatment with teriparatide, anticatabolic drugs are initiated to preserve the gains in BMD, which are otherwise fairly rapidly lost when the drug is stopped.45 Switching from an anticatabolic agent to teriparatide or selecting a patient for this drug as initial therapy is a decision that may be best made by an osteoporosis specialist.
Combination Therapy.
There are small studies that have demonstrated that the combination of 2 anticatabolic agents (alendronate and estrogen or raloxifene) increases bone density more than either single agent alone.46,47 Outcomes of trials using the combination of teriparatide and an anticatabolic agent have suggested that concomitant anticatabolic therapy may limit the increment in BMD (and presumably antifracture efficacy, though not proved) from teriparatide.43,44 No trials of any combinations of therapies with fracture as an end point have been performed. The additional cost and potential for side effects from combination therapy must be weighed against the possible gains. Although combinations of antiosteoporotic therapies may be used in certain cases, the decision to use more than 1 drug is best made by an osteoporosis specialist.
What to Do with Your Patients with Vertebroplasty
Ask
Every patient presenting for vertebroplasty of a low-trauma vertebral compression fracture related to osteoporosis (or presumed osteoporosis) should be asked about current use of calcium, vitamin D, and medications to treat osteoporosis. Because oral bisphosphonates have specific administration instructions, patients currently taking these drugs should be asked how they are taking them and how often they miss a dose. Proper instruction in their use should be given if needed. Patients should also be asked if they have ever been told they have osteoporosis or if they have had a bone density test.
Educate
An encounter to treat a fracture is an important opportunity to educate the patient about the relationship of the fracture to underlying osteoporosis. Often patients will dismiss this notion by stating that the fracture occurred because of a “hard” fall or is simply related to old age. A patient's acceptance of the diagnosis of osteoporosis (and its treatment) may be improved by having the physician treating the fracture emphasize the relationship. Patients undergoing vertebroplasty should be told that their risk of additional fractures is high and that the procedure will not lower that risk but drug treatment of osteoporosis will.
Advise
The treating radiologist should advise both the patient and the primary care physician that appropriate medical evaluation for osteoporosis including bone density testing, if not performed recently, is needed. Referral to an osteoporosis specialist may be advised for premenopausal women and men because a more extensive evaluation of the causes of bone loss may be needed. Referral should also be considered for those with very low bone density, multiple fractures despite therapy, and complex medical problems or for those who have been intolerant of osteoporosis therapies. Patient selection for teriparatide or combinations of drugs is best determined by an osteoporosis specialist.
Conclusion
Patients presenting for vertebroplasty are at high risk for additional fractures and, therefore, should receive appropriate evaluation and treatment to lower the risk of subsequent fractures. The radiologist performing vertebroplasty has the opportunity to improve the care of these patients by asking about osteoporosis diagnosis and current drug therapy, educating the patient about the relationship of the compression fracture to underlying osteoporosis, and advising the patient and referring physician that appropriate evaluation and treatment of osteoporosis are indicated. The information provided here about the evaluation and treatment of osteoporosis will hopefully provide the radiologist with necessary knowledge to close the loop on the postfracture care of patients with osteoporosis whom they encounter.
References
- Copyright © American Society of Neuroradiology