Abstract
BACKGROUND AND PURPOSE: Vestibular schwannoma (VS) is a benign, slow-growing tumor, and radiologic monitoring is an acceptable alternative to surgery in small lesions and in elderly patients. MR imaging with contrast is the study of choice in the follow-up of these lesions. However, gadolinium-based contrast agents have side effects and should be used only when definitely indicated. The purpose of this study was to evaluate the diagnostic accuracy of the constructive interference in steady state (CISS) sequence used without postcontrast sequences for the follow-up imaging of VS.
MATERIALS AND METHODS: MR imaging examinations of 18 patients were retrospectively evaluated by 2 radiologists. VS masses were measured on both CISS and the postcontrast images by each observer. For each patient, the masses were also assessed qualitatively for possible progression between every consecutive study.
RESULTS: Fifty MR images of 18 patients were evaluated. Patients had 1–5 follow-up studies. The mean time interval between the consecutive studies was 23 months (6–55 months). The sensitivity, specificity, and accuracy of the CISS sequence for the detection of progression were 100%. There was good interobserver and intraobserver (CISS and postcontrast) correlation. The CISS sequence had, however, limited sensitivity for the detection of changes in the internal architecture.
CONCLUSIONS: Noncontrast CISS-only technique may be a viable alternative to routine contrast-enhanced sequences for the follow-up of overall lesion size in patients with VS; however, treatment-related changes internal to the tumor are less noticeable using the CISS sequence.
Vestibular schwannoma (VS) is a benign nerve sheath tumor of Schwann cell origin.1 VS is the most frequently seen mass in the cerebellopontine angle (CPA). It constitutes 60%–90% of all CPA tumors and 8%–10% of all intracranial tumors.2,3 This tumor most commonly occurs in patients between 40 and 60 years of age and is more frequent among women.4 Most lesions show a slow growth (approximately 2 mm/year), but some may grow up to 1 cm in a year.4–6 There is no established consensus regarding the treatment of VS; however, when small in size, radiologic follow-up and radiosurgery are considered alternative treatments to surgery, especially in the older age group, who have increased surgery-related morbidity rates.7,8
MR imaging of the temporal bone is currently the study of choice in the diagnosis and follow-up of VS.9–11 In this examination, postcontrast T1-weighted images (T1WI) are routinely used in addition to precontrast T1WI and T2-weighted images (T2WI). In most centers, 1-mm-thick heavily T2WI gradient-echo images such as constructive interference in steady state (CISS) or balanced fast-field echo are also obtained to better assess cranial nerves and intralabyrinthine fluids.12,13 Gadolinium-based contrast agents, which are used in MR imaging, have additional cost and possible side effects. In particular, due to the potential for nephrogenic systemic fibrosis (NSF), these agents should be used with caution in patients with renal insufficiency and only when definitely indicated.7,8
Therefore, it is important to determine whether the use of contrast agents is necessary and justified in the follow-up imaging of VS, especially in the elderly age group, who have decreased glomerular filtration rates (GFRs) and thus are at increased risk of renal dysfunction. Although the necessity of postcontrast imaging for the diagnosis and initial imaging of VSs has been previously investigated, to the best of our knowledge, the comparative accuracy of high-resolution T2WI versus postcontrast T1WI in monitoring change over time has not been evaluated.14,15 This study was designed to evaluate the accuracy of the CISS sequence for the follow-up of patients with VS in comparison with standard contrast-enhanced spin-echo T1WI.
Materials and Methods
Patients
A data base search was performed to identify all patients between March 2003 and March 2008 with imaging diagnoses of VS who had follow-up MR imaging. Patients with the diagnosis of neurofibromatosis type 2 (NF-2) were excluded from the study because their tumor-growth characteristics are different from those of sporadic VS.15–17 Eighteen patients were identified.
Imaging
MR imaging examinations were performed with either a 3T (Allegra; Siemens, Erlangen, Germany) or a 1.5T scanner (Symphony; Siemens), by using a standard head coil. The standard temporal bone protocol included axial T1WI and T2WI, axial and sagittal oblique 3D CISS images, and postcontrast axial and coronal T1WI. The postcontrast sequences were obtained immediately after the injection of 0.2 mL/kg of contrast. The axial spin-echo T1WI were obtained through the internal auditory canal (IAC) with the following parameters: TR/TE, 550/15 ms; 2-mm section thickness with a 0.3-mm intersection gap; NEX, 2; matrix, 256 × 192; FOV, 180. Transverse-oriented CISS 3D imaging was performed with the following: TR/TE, 7.42/3.71 ms; flip angle, 45; NEX, 1; 1-mm section thickness; FOV, 170 mm; matrix, 448 × 352. Fast spin-echo T2WI of the brain were obtained with TR/TE, 3240/100 ms; echo-train length, 15; section thickness, 5 mm; gap, 20%; NEX, 2; matrix, 448 × 223. The imaging protocols were kept stable for each scanner throughout the 5-year period.
Image Evaluation
MR imaging (initial scans and all the available follow-up studies) of these 18 patients was retrospectively and independently evaluated by 2 radiologists (observer 1 was a neuroradiologist with extensive experience in head and neck imaging). The tumor size was graded according to the Koos classification (Koos stage 1, tumor confined to the IAC; stage 2, tumor <2 cm in diameter; stage 3, tumor >2 cm, not compressing the brain stem; stage 4, tumor compressing the brain stem irrespective of its size).18 The tumor size was assessed with diameter measurements as suggested by Fiirgaard et al,19 who reported that diameter measurement was an easier but equally accurate parameter than volume calculation for the comparison of VS size.
The diameters of VS masses were measured in 3 orthogonal planes on both CISS and the postcontrast images by each observer. To obtain a precise and standardized assessment of tumor, we made the 3 measurements as follows: maximum anteroposterior diameter perpendicular to the IAC (AP), maximum transverse diameter parallel to the IAC (TRA), and the maximum-height, superoinferior diameter (SI). The AP and TRA measurements were performed on the axial images, and the SI diameter was measured on the coronal images (reformatted coronal CISS images or direct coronal postcontrast T1WI). For large masses with an extrameatal component, the AP and SI dimensions were measured from the extrameatal part. The measurements on CISS images and postcontrast sequences of the same patient were performed on different occasions to prevent bias; the CISS images were evaluated first, without knowledge of the findings on the contrast-enhanced T1WI. For each observer, the measurements obtained from postcontrast T1WI were accepted as the gold standard. For each consecutive scan, we also made a qualitative assessment in regard to tumor progression, evaluating the change in size (previously measured) and the extent of the tumor. Again the assessment of the progression performed with the postcontrast sequences was taken as the gold standard
The statistical evaluation of measurements was done with the paired-sample t test and Pearson correlation coefficient. Bland-Altman analysis was also performed to assess the interchangeability of measurements obtained from CISS and postcontrast images. The evaluation of possible progression on the CISS sequence in respect to postcontrast images was done with the McNemar test for proportions. The McNemar test and Cohen κ coefficient were used to assess the intraobserver agreement.
Results
Fifty MR images of 18 patients were evaluated. The patient data and imaging information are summarized in Table 1. Of the 18 patients, 7 were men and 11 were women, with a mean age of 55 ± 16 years (range, 25–78 years); 33.3% of the patients were older than 65 years and 77.8% were older than 50 years. The patients had 1–5 follow-up studies with a mean of 1.8 follow-up imaging studies. The mean time interval between the initial study and the last follow-up study was 23 months (with an interval of 6–55 months and a median of 14 months).
Patient data and imaging information
The average size of the tumors was 8.4 × 10.1 × 7.5 mm; tumor size varied between 3.1 × 3.9 × 3.3 mm and 33.1 × 23.6 × 30.4 mm. Among the 18 masses, 11 measured <1 cm in maximum diameter. Seven lesions (39%) were confined to the IAC (Koos stage 1); 44.4% of the lesions (8/17) were Koos stage 2. A lesion that was Koos stage 3 at initial imaging progressed to stage 4 on the follow-up study. Two patients had Koos stage 4 lesions.
Two patients (patients 4 and 13) had prior surgery with residual tumor; and in both patients, the first available study was a postoperative scan. Patient 7 was being followed-up after CyberKnife (Accuray, Sunnyvale, Calif) surgery; therefore, the first available study was obtained after the treatment.
Among 18 patients, tumor progression was detected in 5 by observer 1 on both the CISS and the postcontrast T1WI. Observer 2 identified progression in only 2 patients, both on the CISS and on the postcontrast T1WI. Despite this difference among observers, there was no difference in the detection of progression in the CISS sequence compared with postcontrast T1WI for each individual observer (P = 1.00, McNemar test for proportions). The sensitivity, specificity, and accuracy of the CISS sequence for the detection of progression were 100% for each observer. There was a moderate agreement for the evaluation of progression between the 2 observers (κ = 0.531, P < .001). A single patient (patient 4) showed a slight decrease in size in the fourth follow-up study. This tumor regression was detected by measurements of both observers.
Overall, the measurements obtained from the CISS images were slightly smaller than the ones obtained on postcontrast T1WI, but the difference of the mean values was <0.5 mm (Table 2). There was good correlation of the sets of CISS-postcontrast measurements for each observer (r = 0.952–0.995, P < .001). Bland-Altman plots were used to assess differences between the measurements (CISS and postcontrast) for each orthogonal plane and for each observer (On-line Fig 1). The plots revealed that the measurements obtained from the CISS and postcontrast images were within the observed limits of agreement, indicating that the 2 measurement methods could be used interchangeably.
Comparison of measurements performed on CISS and Post Gad images
The first observer's measurements were slightly higher than those of the second observer, but again the difference between the mean values was <0.5 mm (Table 3). There was a good degree of correlation between the measurements performed by the 2 observers (r = 0.938–0.989, P < .001).
Interobserver variations between the measurements performed by 2 observers
In 2 patients, the heterogeneous internal architecture of the IAC masses was less appreciable on the CISS sequence than on the contrast-enhanced T1WI. One the patient had the VS treated by CyberKnife, in whom the internal heterogeneity of the tumor that appeared on the follow-up study most likely reflected treatment-related changes. The mass of the other patient (patient 16) showed decreased heterogeneity, with a more solid appearance on the follow-up imaging (only appreciated on the postcontrast T1WI and not with CISS images). However, the finding was accompanied by increased size, consistent with tumor progression, detected on both postcontrast T1WI and CISS images.
Discussion
VS is a relatively common tumor with a clinical incidence of 10–15 per million.20 Most lesions occur in patients between 40 and 60 years of age.21 The mean age of the patients in this study was 55 years (16.07 SD). Three of our patients were younger than 30 years of age; however, their hospital charts and available information revealed no findings suggestive of NF-2. VS has been reported to occur slightly more frequently in women, which is in agreement with a slight female predominance (11/18 patients) in our group.21
The VSs are typically slow-growing histologically benign tumors.1,22 Studies investigating the natural history of sporadic VS reported a growth rate of <2 mm/year.6,23,24 Approximately 43%–80% of these lesions show no growth on follow-up,25,26 as was the case in 13 (72.2%) of our patients. Spontaneous regression, with an overall incidence of 5%, has also been described.26–28 Similarly, the lesion of our patient 4 showed a slight decrease in size without any treatment in the fourth follow-up study performed 3.5 years after the initial imaging. Despite the benign course of VS, the classic management of these lesions has been traditionally surgical.29 However, with the development of imaging and radiation treatment technologies, radiologic observation with serial imaging studies or treatment with stereotactic radiation therapy (such as gamma knife surgery) has become an acceptable alternative.5,6,23–25,27,28,30
The radiologic surveillance is especially preferred in elderly patients, in patients with small tumor size or with minimal symptoms, and in patients who are poor surgical candidates.5,23,24,27,28 Contrast-enhanced T1WI is the established standard technique for this follow-up imaging, and an increasing number of patients are being followed-up with this technique.5,9,25 In addition, possible recurrence or growth of a residual tumor following VS surgery is also monitored with contrast-enhanced MR imaging.29,31 Although the postcontrast images are routinely obtained for each of the above-mentioned circumstances and in every single subsequent follow-up study, the necessity of contrast use in follow-up imaging has never been questioned.
The gadolinium compounds that are used as contrast agents in MR imaging are substances with potential side effects and risk of allergies. Moreover, the recent awareness of the NSF disorder requires additional caution for the use of gadolinium chelates.7,8 NSF is a rare but potentially serious acquired systemic disease, which occurs when gadolinium-based contrast agents are administered to a patient who has either severe or acute renal impairment (especially when GFR is <20 mL/min).7,32,33
Several investigators have evaluated high-resolution T2WI such as 3D CISS as an alternative to postgadolinium imaging.13–15,34 The CISS sequence is a T2* weighted refocusing 3D gradient-echo sequence.12,13 This sequence has a high spatial resolution, allowing good contrast between CSF and other structures (such as nerves, bone, and soft tissues), and has also been reported to be highly accurate in detecting lesions of the CPA-IAC.12,13,35 The value of the CISS sequence used alone as a replacement for contrast-enhanced T1WI sequences has been assessed for the initial detection of VS.13–15,34,35 Hermans et al34 showed that by using CISS alone, even small VS lesions could be detected with a sensitivity of 89%–94% and a specificity of 94%–97%. Stuckey et al15 also reported that CISS images were highly accurate in the detection of VS with a sensitivity of 94%–100% and a specificity of 93.5–98.5% and that CISS sequence could be used as a suitable screening study for VS.
Nevertheless, both studies and other reviews also mentioned certain limitations of the CISS sequence when used alone for screening purposes.15,34,36 The lack of enhanced images may result in false-positive findings due to the clustering of nerves and vascular structures as well as false-negative interpretations for small inflammatory lesions of the labyrinth and meninges.10,15,34,36 The above-mentioned limitations of the CISS sequence are not valid in the setting of follow-up imaging for VS because the lesion is typically already diagnosed and delineated on a prior (initial) enhanced MR imaging of the temporal bone. In that setting, the question we addressed is whether the lack of contrast images hampers the detection of progression.
Our study demonstrates that there is indeed no difference in the detection of progression by using the CISS sequence alone compared with postcontrast sequences (Figs 1 and 2). This study is, of course, somewhat limited due to the small size of patient group and especially due to the small number of patients demonstrating progression in the follow-up. However, conservative management is generally contemplated when the expectation for progression is relatively low (small tumor size, elderly patients, etc), so the low rate of progression in our group is, in fact, an expected finding.5,23,24,27,28 The relatively short interval of follow-up imaging in the study (with a mean follow-up period of 23 months) may account for this low occurrence of progression because the VSs are known to have slow growth rates,6,22,23 and rapid progression following a relatively long quiet period has also been reported.5 This was the case for one of our patients who demonstrated progression in her fifth follow-up study (4.5 years after the initial study). However, all the remaining patients had evidence of progression in their first follow-up imaging (performed 6–24 months after the initial scanning), which is consistent with the results of Stangerup et al,26 who reported that in most VSs, the growth occurs in the first 5 years after the diagnosis and especially in the first year. Prospective studies with a larger number of patients and those involving growing tumors are needed to demonstrate better the accuracy of the CISS sequence versus postcontrast T1WI. Our preliminary results appear to encourage the use of side effect−free CISS sequences for the monitoring of VS.
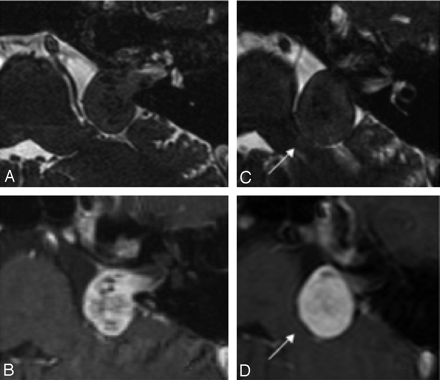
A and B, Axial CISS (A) and postcontrast T1WI (B) of the left ear in a patient with Koos stage 3 VS. C and D, The follow-up imaging with CISS (C) and postcontrast T1WI (D) performed 1 year later demonstrates interval growth of the lesion with new brain stem compression (arrow), consistent with progression to Koos stage 4. Note that loss of internal heterogeneity is less well appreciated on the CISS sequence (C) than on the postcontrast image (D).
A and B, Koos stage 2 VS on the axial CISS image (A) and postcontrast T1WI (B). C and D, The follow-up imaging reveals minimal progression of the lesion with increased protrusion into the cerebellopontine cistern, both on the CISS (C) and postcontrast T1WI (D).
Most of our patients had small lesions (83.3% had stage 1 or 2 lesions), but the paucity of larger lesions is not a real limitation of this study. Large tumors, especially those associated with brain stem compression, are usually referred to surgery without a period of observation unless the patient has a contraindication for surgery.5,25,27
Although there is a good correlation of the measurements performed by the 2 observers, the second observer had a significantly reduced detection of progression compared with the first observer on both the postcontrast images and CISS images, resulting in moderate correlation in qualitative progression assessment. This finding may be related to the difference in experience of head and neck imaging between the observers. The cases that were falsely labeled as stable by observer 2 had only minimal growth (Fig 2), which was detected by observer 1, who had extensive experience in head and neck imaging (Table 1).
Two of our patients had previously undergone surgery, with small residual tumors (Fig 3). The evaluation of the CISS images alone was more challenging for this particular case, and the initial diagnosis of a residual tumor would not have been possible without the postcontrast images. However, with the contrast-enhanced images of the first scan available during subsequent follow-up, the CISS sequence alone was sufficient for the follow-up evaluation. This finding is obviously of limited value because it could not be verified by other similar cases. Nevertheless, it proves that nonenhanced imaging might also be an option for the monitoring of postoperative patients.
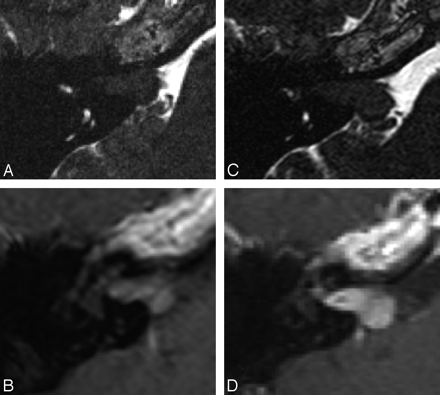
A−C, Postoperative MR imaging of a patient with right-sided VS. The axial T1WI (A), postcontrast T1WI (B), and CISS (C) image demonstrate postoperative changes on the right and a small residual lesion (arrow) within the cerebellopontine angle. D−F, The residual mass (arrow) is stable in the follow-up T1WI (D), postcontrast T1WI (E), and CISS imaging (F) performed 2 years later.
Another patient in our group received treatment with CyberKnife (Fig 4). On the second MR imaging, the mass demonstrated increased volume with increased heterogeneity and necrosis, likely representing the temporary enlargement (or transient swelling) in response to radiation treatment.37–39 The change in internal structure was much better appreciated on the postcontrast images than on the CISS sequence (Fig 4). The overall increase in size of the mass was accepted as progression by the first observer during the qualitative assessment when indeed it may have reflected a positive response to treatment.37–39 This finding demonstrates that the noncontrast CISS-only approach may be of limited value in the evaluation of patients following radiation treatment.
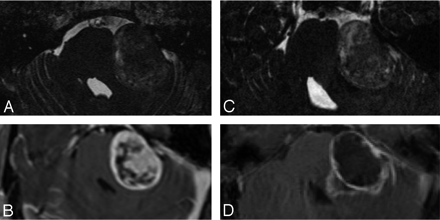
Imaging appearance of a Koos stage 4 VS treated with CyberKnife. The CISS image (A) and postcontrast T1WI (B) demonstrate a large heterogeneous mass compressing the brain stem. The CISS image (C) and postcontrast T1WI (D) of the follow-up study reveal an interval increase in the size of the mass. The increased internal heterogeneity is not well reflected in the CISS image (C).
Similarly, in another patient, the tumor became more compact with decreased internal heterogeneity on follow-up; however, this finding was again less well-appreciable in the CISS sequence (Fig 1). Untreated VSs are known to demonstrate internal heterogeneity and intratumoral cysts, especially when the tumor increases in size.2,21,22 This cyst formation has a reported incidence that varies from 7.6% to 24%.40 Because the central degeneration is a part of the natural history of some VSs, the lack of information regarding the internal structure of the lesions may be a limitation of this noncontrast imaging technique.
Conclusions
Our study demonstrates that the noncontrast CISS-only technique may be a suitable alternative to the routine contrast-enhanced studies for the follow-up of patients with VS, especially in patients with renal failure. However, the CISS sequence has a low sensitivity for the detection of changes in the internal architecture, which may limit its use for the follow-up of patients after radiation treatment. Future studies incorporating larger sample sizes are required to evaluate the utility of CISS sequences for the follow-up of patients with VS.
Footnotes
indicates article with supplemental on-line figure.
References
- Received September 4, 2008.
- Accepted after revision November 22, 2008.
- Copyright © American Society of Neuroradiology