Abstract
BACKGROUND AND PURPOSE: Carotid angioplasty with stent placement (CAS) is an optional treatment for significant carotid stenosis. Cerebral vasoreactivity (CVR), representing the reserve capacity of cerebral perfusion, usually decreases in patients with severe carotid stenosis. This study aimed to investigate the relationship between the baseline CVR assessed by functional MR imaging (fMRI) and the changes in cerebral blood flow (CBF) after CAS.
MATERIALS AND METHODS: Fourteen patients with at least 70% unilateral carotid stenosis underwent CAS. Baseline CVR was evaluated by fMRI a under breath-holding paradigm. CBF was assessed by dynamic susceptibility-weighted contrast-enhanced MR imaging before and 3–5 days after CAS. The lateral index (LI) was defined as (n − L) / (n + L), where n and L represent the number of activated voxels in fMRI on the normal and lesion hemispheres, respectively.
RESULTS: No subject had clinical evidence of hyperperfusion syndrome. The LI represented baseline CVR. Patients were divided into normal (LI < 0, n = 6) and impaired (LI > 0, n = 8) CVR groups. The CBF on the normal and lesion sides was calculated separately. CBF increment on the lesion side after CAS was significantly higher in the impaired CVR group than that in the normal CVR group (P = .035). There was a significantly positive correlation between CVR impairment and the CBF increment (P = .026).
CONCLUSIONS: fMRI could be a reproducible tool in evaluating CVR. After CAS, early CBF changes on the lesion side are more prominent in patients with impaired CVR. Baseline CVR might predict early CBF increase after CAS.
Significant carotid stenosis or occlusion increases the incidence of ischemic stroke or transient ischemic attack (TIA) by compromising cerebral hemodynamics.1,2 Patients with critical extracranial carotid stenosis often benefit from carotid interventions, including carotid endarterectomy and carotid angioplasty with stent placement (CAS), to prevent subsequent ischemic events.3–6 Hyperperfusion syndrome, with common symptoms including unilateral headache, seizures, and intracerebral hemorrhage (ICH),7–9 usually leads to a disastrous outcome if it occurs after carotid interventions. The incidence of ICH after carotid endarterectomy is 0.3%–1.8% but is relatively higher (≤4.4%) after CAS.7,10–13 Several factors, including age, extremely critical carotid stenosis (≥90%), perioperative hypertension, and poor collateral circulation of the brain, are thought to be associated with hyperperfusion syndrome.14 Recent studies have considered impaired cerebral vasoreactivity (CVR) as one of the predictive factors for hyperperfusion syndrome.15,16
CVR may represent the reserve capacity of cerebral perfusion and is often decreased in patients with severe carotid stenosis.17,18 When considering the degree of stenosis, previous studies have suggested that CVR might reflect the hemodynamic status better.19,20 In addition, several investigations have indicated that impaired CVR was an independent risk factor for ischemic stroke or TIA in patients with critical carotid stenosis or occlusion.21–23 Although the specific relationship between the changes in cerebral perfusion and baseline CVR remains uncertain, the incidence of hyperperfusion syndrome after carotid interventions is slightly higher in patients with decreased CVR.16,24 Hence, evaluation of the hemodynamic status by measuring CVR is usually recommended in patients with carotid stenosis.
CVR can be assessed by transcranial Doppler sonography, single-photon emission CT (SPECT), or MR angiography after administration of vasodilatory stimuli (eg, acetazolamide injection or carbon dioxide [CO2] inhalation).25–28 However, there are very few published reports on the evaluation of CVR by functional MR imaging (fMRI).29,30 Blood oxygen level–dependent (BOLD) signal-intensity activation revealed by fMRI is sensitive during increased cerebral blood flow (CBF) and is often used for detecting the changes in cerebral perfusion under various physiologic conditions. The aim of this study was to evaluate the relationship between the baseline CVR assessed by fMRI and the changes in CBF for patients undergoing CAS.
Materials and Methods
Patients
Between January 2007 and September 2007, 14 patients with unilateral internal carotid artery (ICA) stenosis (≧70% according to the North American Symptomatic Carotid Endarterectomy Trial criteria) who underwent subsequent CAS treatment were enrolled for this study (Table 1). Exclusion criteria included interventional coronary or peripheral artery treatment in the past 30 days, stroke within the past 3 weeks, a previous stroke producing (≥ one-third) middle cerebral artery (MCA) territory infarction, significant (≧50%) intracranial stenosis, allergy to iodinated or gadolinium-based contrast medium, and inability to perform a breath-holding task cooperatively. All the patients underwent a series of tests, including brain CT, color-coded carotid duplex and transcranial Doppler sonography, and digital subtraction angiography, before undergoing CAS. Blood pressure was carefully monitored during the periprocedural period, and the averaged mean arterial pressure (MAP) on the day before intervention was 92.81 ± 6.58 mm Hg.
Demographic data of 14 subjects
Written informed consents were obtained from all the patients, and the study was approved by the local institutional review board.
Clinical Hyperperfusion Syndrome
Clinical hyperperfusion syndrome was defined as severe ipsilateral headache, seizure, or intracerebral hemorrhage after CAS.
MR Imaging Acquisition
Before intervention, patients were asked to hold their breath while their baseline CVR was determined by BOLD MR imaging. Dynamic susceptibility-weighted contrast-enhanced MR imaging was performed both before and 3–5 days after CAS for evaluating cerebral perfusion.
MR imaging studies were conducted on a 1.5T scanner (Gyroscan Intera; Philips, Best, the Netherlands). The scanning protocols included anatomic sequences, diffusion imaging techniques, perfusion techniques, and BOLD MR imaging studies. The anatomic images included axial T1-weighted images (spin-echo; TR/TE = 449/12 ms), axial T2-weighted images (fast spin-echo; TR/TE = 4000/90 ms, echo-train length = 17), and fluid-attenuated inversion recovery (FLAIR; TR/TE/TI = 9416/90/2200 ms). Twenty axial sections (width = 5 mm and intersection gap = 1.5 mm) were acquired to cover the whole brain. All the imaging studies were performed with the same dimensions (ie, number of sections, section thickness, and locations). Diffusion imaging used a single-shot spin-echo echo-planar imaging sequence (TR/TE = 2812/60 ms; b-values = 0, 500, and 1000 s/mm2).
For the dynamic BOLD study, the breath-holding paradigm was modified according to that specified in previous studies.31,32 Before the breath-holding task, patients were required to breathe naturally for at least 6 minutes. During fMRI scanning, patients were asked to hold their breath for 15 seconds and then to breathe naturally for the next 45 seconds. This cycle was repeated 4 times. A respiratory belt was fastened across each patient's chest to ensure that the patients followed the breathing instructions properly. BOLD MR imaging was performed by using a T2-weighted single-shot gradient-echo echo-planar imaging sequence with the following parameters: TR/TE/flip angle (FA) = 3000/50 ms/90°, matrix size = 112 × 84, and FOV = 192 × 192 mm. During the breath-holding procedure, 80 dynamic measurements were obtained with a total scanning time of 4 minutes.
The parameters for the dynamic susceptibility-weighted contrast-enhanced MR imaging single-shot gradient-echo echo-planar imaging sequence were as follows: TR/TE/FA = 1500/40 ms/55°, matrix size = 112 × 128; and FOV = 240 × 240 mm. Sixteen milliliters of gadolinium-diethylene-triamine pentaacetic acid (Magnevist; Schering, Berlin, Germany) was injected by using a power injector with an injection rate of 4 mL/s.
Image Interpretation and Analysis
Determination of Region of Interest.
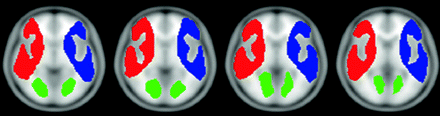
The T1-weighted images of each subject were normalized to the standard Montreal Neurological Institute (MNI) brain template by using Statistical Parametric Mapping software (SPM2 software; Wellcome Department of Imaging Neuroscience, London, UK). The normalized images were then averaged from the data for all the patients to determine the region-of-interest template. The regions of interest were manually placed in bilaterally symmetric regions of the MCA territory by an experienced neurologist. In addition, regions of interest were also placed in the occipital white matter for reference (Fig 1). Subsequently, both BOLD and perfusion MR imaging results were normalized to the MNI space and averaged across the complete MCA territory by using our region-of-interest template (Fig 1). The relative perfusion data of the MCA region were then normalized to that of the occipital white matter region, assuming that the occipital white matter perfusion remained stationary after intervention.
Colored map of the region-of-interest template (selected sections). Blue and red represent the left and right MCA territories, respectively. Occipital white matter, which is shown in green, is used as a reference region.
Data Analysis of CVR.
All BOLD images were spatially smoothed with a gaussian kernel (full width at half maximum = 5 mm) and spatially normalized to the MNI template by using SPM2 software before data processing. To explore the voxels with significant BOLD signal-intensity change, which was caused by breath-holding, we applied a correlation analysis to individual whole-brain volume, and the correlation coefficients thus obtained were transferred to t-scores. The reference time curve of each subject was obtained by averaging the time curves of the whole-brain tissue. Voxels with significant BOLD signal-intensity changes were determined with a threshold of t > 3.21 (P < .001, uncorrected). The number of statistically significant voxels was calculated for the regions of interest of the left and right MCA territories. The lateral index (LI) was defined as (n − L) / (n + L), where n and L represent the number of activated voxels in fMRI of the normal and lesion sides, respectively. Hence, a positive LI corresponded to a decreased CVR of the lesion side before CAS.
Data Analysis of Perfusion.
Perfusion images were analyzed by using the Nordic Image Control and Evaluation software (nordicICE; NordicImagingLab, Bergen, Norway). Arterial input functions were chosen from the MCA to quantify relative CBF by generating CBF maps using the singular value decomposition deconvolution algorithm.33 The relative CBF values obtained in the MCA regions were then divided by the mean value of the ipsilateral occipital white matter region, and the ratio represented a normalized CBF value. The changes in the normalized CBF of either side of the brain were semiquantified by a CBF index calculated as follows: (CBF after CAS) / (CBF before CAS). A CBF index value of >1 represented increased blood flow after CAS.
Statistical Analysis
Descriptive statistics were presented as mean ± SD. The Student t test and the χ2 test were applied for comparison. Values with P < .05 were considered to be significant. Scattergrams were constructed, and correlation analysis was performed by applying the Pearson correlation coefficients. All statistical operations were performed by using a commercially available software package (SPSS for Windows 13.0; SPPS, Chicago, Ill).
Results
The demographic data of 14 patients are listed in Table 1. All subjects were men, and the mean age was 73.5 ± 6.58 years (range, 56–82 years). Before CAS, 8 patients had previous cerebral infarctions and 3 patients had transient ischemic attacks. The other 3 patients had severe posture-related dizziness without a definite ischemic event. All the patients who had previously experienced strokes showed good functional recovery with modified Rankin Scale (mRS) scores ≤2. All patients underwent a successful CAS procedure, and none developed clinical hyperperfusion syndrome. No infarcts developed in any patient after CAS, as was demonstrated by MR imaging.
Baseline CVR
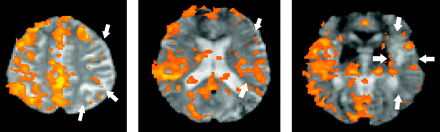
The patients were divided into 2 groups according to the LI: the impaired CVR group (LI > 0) and the normal CVR group (LI < 0). Before intervention, the CVRs of the lesion side were relatively decreased in 8 patients (LI = 0.19 ± 0.16) (Fig 2); the other 6 subjects had normal CVRs on the lesion side (LI = − 0.09 ± 0.12).
BOLD signal intensity on fMRI under a breath-holding paradigm in a patient with left ICA stenosis (subject 2). The BOLD signal intensity is relatively decreased on the left side (the lesion side, arrows). The LI of this patient is 0.53, which indicates an impaired baseline CVR.
Comparison between the 2 Groups
The clinical condtions of the patients before intervention were compared between the impaired and the normal CVR groups. There was no statistically significant difference in terms of mean age, severity of internal carotid artery (ICA) stenosis, MAP, and baseline mRS score before CAS (Table 2).
Comparison between patients with impaired and control CVR
Relationship between Baseline CVR and the Changes in CBF after CAS
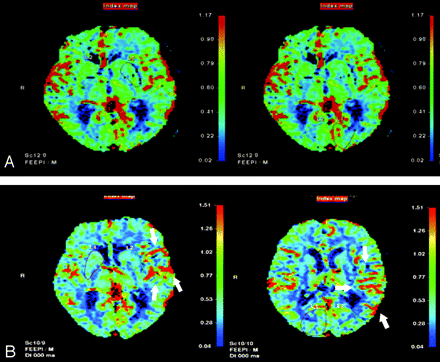
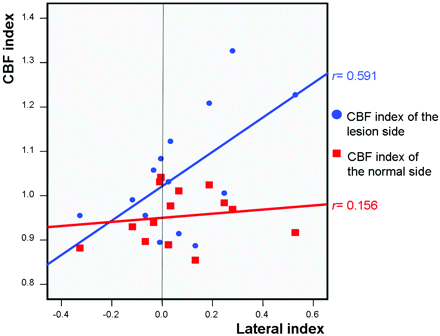
The CBF index was used to represent the changes in CBF (Fig 3) after CAS. The CBF indexes for the lesion and normal sides were estimated separately. The values of the CBF index for the normal side were similar in the normal and impaired CVR groups (Table 2, P = .728). On the other hand, the value of the CBF index on the lesion side was significantly higher in patients with impaired CVR than in patients with normal CVR (Table 2, P = .035). There was significantly positive correlation between the baseline CVR and CBF index on the lesion side (Fig 4; r = 0.591, P = .026), but no significant correlation was observed on the normal side (Fig 4; r = 0.156, P = .728).
CBF on perfusion MR imaging before (A) and after (B) CAS for the left ICA of subject 2. Before intervention, the CBF on the left side is lower than that on the right side. After CAS, CBF on the left side increases (arrows) and is higher than that on the right side. The CBF index of the lesion (left) side is 1.23, whereas that of the normal side is 0.92.
Relationship between baseline LI of CVR and CBF indexes. The dots indicate the LI and CBF index of the lesion side of each patient; the squares indicate the LI and CBF index of the normal side of each patient. Fit lines are drawn by a linear regression method.
Discussion
Previous reports have suggested impaired CVR as a predictor of hyperperfusion after carotid intervention, including carotid endarterectomy and CAS.15,16,24 Hosoda et al15 observed that in patients who had undergone carotid endarterectomy for carotid stenosis, the ipsilateral CBF increment was more significant in the impaired CVR group than that in the normal CVR group on the first postoperative day. Kaku et al16 stated that the values of CVR before treatment were associated with hyperperfusion phenomenon after CAS. In this study, the changes in CBF on the lesion side after intervention were significantly higher in patients with impaired CVR (Table 2, P = .035). Further analysis revealed positive correlation between the baseline CVR and CBF changes (Fig 4). These findings imply that a worse baseline CVR corresponds to a greater increase in CBF on the lesion side after intervention. Although none of our patients had clinical hyperperfusion syndrome, in which strict control of their blood pressures (MAP = 92.81 ± 6.58 mm Hg) and intensive monitoring during peri-CAS period might play important roles, relative cerebral hyperperfusion was still demonstrated on the lesion side in patients with impaired CVR (Table 2).
CVR is an important indicator of cerebral hemodynamic integrity and autoregulation. When CVR decreases, cerebral hemodynamic reserves start to decline.34,35 The mechanism of hyperperfusion has been suggested as extreme vasodilation due to poor hemodynamic reserve from chronic cerebral ischemia distal to the critical carotid stenosis.15 Under the circumstance of compromised autoregulation, the increment of perfusion after reperfusion therapy might be more vigorous. This explains why there is a positive correlation between impaired CVR and increased CBF in our patients. On the other hand, prominent CBF increase may indicate the efficacy of carotid intervention, as long as no complications occur. Thus, patients with impaired CVR may have more benefits from reperfusion therapy for carotid stenosis than those with normal CVR. Hence, CVR should be considered as an indicator for selecting patients for carotid intervention and in determining the timing of the procedure, especially in patients without symptoms.
In addition to hyperperfusion, CVR is also crucial in identifying high-risk patients who may experience subsequent ischemic events. Although the major cause of stroke in patients with severe arterial stenosis or occlusion is thought to be thromboembolism, the hemodynamic compromise also plays an important role.23,36 A recent study retrospectively analyzed the patterns of infarction in patients with carotid occlusion and hemodynamic failure, and the data suggested a synergistic effect between thromboembolic and hemodynamic mechanisms for the ischemic stroke.37 Furthermore, impaired CVR may predict stroke or TIA risk in patients with either asymptomatic or symptomatic carotid stenosis.21,22 However, whether CVR could be improved after carotid intervention and/or the incidence of stroke could decrease with CVR restored needs further investigation. In the present study, none of the patients had new infarcts in the early stage after CAS. The change of hemodynamic status and its relationship with the prognosis after intervention have to be followed up longitudinally.
As an indicator of cerebral hemodynamic reserve, CVR is evaluated after applying vasodilating stimuli. Among the several methods of inducing vasodilation, CO2 (3%–6%) inhalation may be intolerable to elderly patients and could be dangerous in patients with chronic obstructive pulmonary diseases. Intravenous injection of acetazolamide is invasive and not universally applicable. On the other hand, the breath-holding task is an optional and reliable way for assessing CVR.31,38 Our subjects tolerated breath-holding conditions well. For better cooperation, all of them practiced this paradigm several times before evaluation. We believe that instead of CO2 inhalation or injection of acetazolamide, breath-holding is easier to perform during MR imaging.
According to previous studies, transcranial Doppler sonography and SPECT are the most routinely used tools in evaluating CVR when the clinical application of positron-emission tomography is limited due to its expensive cost and limited popularity. Transcranial Doppler sonography is an easily available tool wherein CVR is represented by the percentage of mean velocity increase after administration of vasodilatory stimuli.25 However, transcranial Doppler sonography is also highly technique-dependent and cannot be applied in patients with a poor temporal window. Moreover, critical carotid stenosis may result in the absence of any MCA signal intensity.39 Hence, transcranial Doppler sonography is not a very practical tool in assessing CVR in patients with critical carotid stenosis. Measurement of increased CBF by SPECT analysis after administration of vasodilating agents remains the most popular method to interpret CVR. The advantage of SPECT is that it can be used to calculate blood flow quantitatively. However, this method is relatively time-consuming, has poor spatial resolution, involves radiation load, and is comparatively more invasive than fMRI.
Recently, the use of BOLD signals on fMRI combined with a vasodilatory stimulus has become an alternative method to assess CVR.30 The benefits of fMRI include its rapid approach, good safety, and better spatial resolution. To date, a few studies present the correlation between transcranial Doppler sonography and fMRI.29,30,40 Although the results of these studies suggest that CVR data obtained from the 2 methods are only moderately correlated, fMRI additionally provides a semiquantitative estimation of CVR. In addition, there are certain characteristic features in this study: 1) The paradigm for fMRI is the breath-holding task, which is more tolerable and easily synchronized compared with other motor tasks (eg, moving fingers). This is important because most patients with severe carotid stenosis are elderly and may have functional impairments due to previous strokes. Breath-holding may also activate a more generalized hemodynamic change on fMRI rather than only a regional blood flow alteration while performing simple motor tasks. 2) The regions of interest include serial sections of functional mapping and total active voxels close to the whole MCA territory. Therefore, the estimation of BOLD signals can be more sensitive and comprehensive.
To focus on the CVR on the lesion side, the difference in activated BOLD signals between hemispheres is divided by the summation of all BOLD signals to create the ratio termed “LI.” This ratio reflects the relative condition of the hemodynamic reserve. A positive LI indicates that BOLD signal-intensity activation is less on the lesion side than on the normal side, whereas a negative LI implies larger hemodynamic response to vasodilatory stimuli on the lesion side. During the process of carotid stenosis, collateral circulation around the circle of Willis may develop with time. Some of our subjects had better CVRs on the lesion side (negative LI). This may indicate abundant collateral flow, which compensates for the cerebral hemodynamics compromised by the ipsilateral carotid stenosis. On the other hand, positive LI may indicate the relatively impaired status of CVR on the lesion side.
We used perfusion MR imaging to monitor the changes in CBF before and after CAS. Although perfusion MR imaging is a semiquantitative tool and might underestimate the magnitude of perfusion changes, the data of CBF on perfusion MR imaging correlates well with SPECT.41 It also provides better anatomic information than SPECT or transcranial Doppler sonography. Moreover, in this study, we estimated the CBF on the basis of nearly complete MCA territory. For calculating CBF, first the CBF of the regions of interest was corrected with reference to the CBF of the occipital white matter. Next, we compared the CBF before with that of after intervention to develop a CBF index. This CBF index may precisely represent the proportion of perfusion change over the MCA territory. After CAS, cerebral perfusion may globally increase. Hence, using the CBF index would help us in delineating the localized CBF changes on the MCA territory.
Our study has certain limitations. First, the sample size was small. Although the degrees of stenosis between 2 groups were not significantly different, whether the stenotic severity has certain influences on CVR or CBF needs to be carefully interpreted. Second, we have discussed only the early stage of CBF changes after the intervention. A longitudinal study with more patients would be required to elucidate more comprehensive understanding of the relationship between CVR and cerebral perfusion.
Conclusions
fMRI with the breath-holding paradigm is a safe, time-saving, and reproducible tool in assessing CVR. In patients with impaired CVR, the early increment of CBF on the lesion hemisphere after CAS is positively correlated with CVR decrease. Further investigations are required to delineate the influence on CBF change by baseline CVR in patients with severe carotid stenosis.
Footnotes
This work was supported by the Medical Research Project, Chang Gung Memorial Hospital (Grants CMRPG350732 and CMRPG340203).
References
- Received December 28, 2008.
- Accepted after revision February 11, 2009.
- American Society of Neuroradiology