Abstract
BACKGROUND AND PURPOSE: CBV is a key parameter in distinguishing penumbra from ischemic core. The purpose of this study was to compare CBV measurements acquired with standard PCT with ones obtained with C-arm CT in a canine stroke model.
MATERIALS AND METHODS: Under an institutionally approved protocol, unilateral MCA strokes were created in 10 canines. Four hours later, DWI was used to confirm the presence of an infarct. CBV maps acquired with PCT were compared with ones acquired by using C-arm CT. Three experienced observers, blinded to the technique used for acquisition, evaluated the CBV maps.
RESULTS: An ischemic stroke was achieved in 9 of the 10 animals. Areas of reduced CBV were detected in 70%–75% of the PCT studies and in 83%–87% of the C-arm CT examinations, with false-positives in 1.7% and 3.3%, respectively. False-negatives were found in 25% of the PCT and 12.2% of the C-arm CT studies. In all studies, there was a significant difference between the absolute CBV values in normal and abnormal tissue (P < .005) and no significant difference between PCT and C-arm CT CBV values in either the normal or the abnormal parenchyma (P > .05).
CONCLUSIONS: CBV measurements made with C-arm CT compare well with ones made with PCT. While further work is required both to fully validate the technique and to define its ultimate clinical value, it appears that it offers a feasible method for assessing CBV in the angiography suite.
Abbreviations
- ACA
- anterior cerebral artery
- CBF
- cerebral blood flow
- CBV
- cerebral blood volume
- DWI
- diffusion-weighted imaging
- DSA
- digital subtraction angiography
- FN
- false-negative
- FP
- false-positive
- ICA
- internal carotid artery
- MCA
- middle cerebral artery
- MTT
- mean transit time
- PCT
- perfusion CT
- TP
- true-positive
- VA
- vertebral artery
Every year almost 800,000 people have a stroke in the United States; approximately 75% of these are first attacks.1 Imaging protocols suitable for distinguishing nonviable from viable brain tissue are feasible with both CT and MR imaging; MR imaging is considered superior to CT in the early detection of acute stroke and is equally able to detect intracranial hemorrhage.2 A recent study, however, demonstrated that PCT with adequate coverage is comparable with advanced MR imaging measurements of the ischemic core/penumbra mismatch.3 PCT can also be used to predict the final infarct volume in patients with stroke with persistent occlusions4 and to assess the risk of subsequent infarction of normal-appearing regions on noncontrast CT.5 Despite these observations, we believe that CT and MR imaging should be considered complementary modalities in acute stroke imaging and their use should be applied in individual patients according to the specific clinical situation.6
CBV is the key parameter in estimating the viability of brain tissue following an ischemic event and, thus, is critical in any scheme aimed at allowing one to differentiate penumbra from ischemic core. The ability to monitor brain viability by assessing CBV during a therapeutic intervention in the same environment (eg, the angiography suite) without the need for patient transportation for another technique would be desirable, especially in instances in which an intervention lasts more than several hours.
With C-arm CT, it is now feasible to obtain CT-like images in the angiography suite, which are sufficient to detect some intracranial hemorrhages.7,8 This technique also provides images that allow the visualization of endoluminal devices and their relationship to the arterial wall and lumen more clearly than those provided by conventional 2D and 3D DSA.9 The current image quality of C-arm CT is, however, not adequate for its use as an initial screening technique to exclude hemorrhage. As image quality of C-arm CT improves, however, triage in the angiographic suite by performing a noncontrast CT to exclude hemorrhage and then assessing CBV to establish brain viability should add incremental value by saving time between an initial assessment in 1 technique (eg, PCT or MR imaging) and initiation of treatment in another by using x-ray fluoroscopy and DSA.
With its current temporal resolution, C-arm CT is not, however, useful in assessing CBF and MTT. It does, nevertheless, provide adequate spatial resolution and contrast sensitivity for the measurement of CBV. In earlier work, we demonstrated that such measurements are very comparable with ones made by using standard PCT.10 The purpose of this study was to test, in a canine stroke model, the hypothesis that C-arm CT measurements of CBV in an area of ischemia would correspond well with ones made by using standard PCT.
Materials and Methods
Stroke Preparation
Under an institutionally approved animal protocol, 10 canines were included in the study. After induction with propofol (2–4 mg/kg), general endotracheal anesthesia was maintained with isoflurane 1%–3%. Constant monitoring of heart rate, O2 saturation, and end-tidal CO2 was performed throughout the experiments. For autologous clot formation, a series of 6F sheath dilators was filled with autologous blood mixed with bovine thrombin and incubated at room temperature for 1 hour. Depending on the variation in the individual animal's vascular anatomy, selective catheterization of either an ICA or a VA was performed by using access from 1 common femoral artery. In animals with large and relatively straight ICAs at the skull base, a 4F guiding catheter was used to first deliver short segments of thrombus directly into the ICA. After delivery of 4–6 pieces of the thrombus, the same number of pieces of 5.0 silk suture, cut to lengths of 3–5 mm, was injected through the same catheter. Serial DSAs were performed to monitor the status of the embolizations. In animals with small or extremely tortuous ICAs at the skull base, a 4F guiding catheter was placed into the cervical segment of 1 VA. Through this, a microcatheter was advanced over a guidewire until it was positioned near the origin of the ipsilateral MCA. Through this microcatheter, multiple aliquots of microfibrillar collagen (Avitene; Bio-Medicine, Houston, Texas) were injected until there was a significant decrease in flow through the ipsilateral MCA. In an attempt to prevent recanalization of the occluded arteries, a single detachable coil was placed into the origin of either the ICA or the VA at the end of the embolization procedure.
DWI
Animals were carefully monitored for 4 hours after embolization, after which each animal underwent DWI on a 1.5T system (Signa HDx; GE Healthcare, Waukesha, Wisconsin) to confirm the presence or absence of cerebral infarction. The parameters for the DWI sequence were the following: TR/TE, 10,000/99.8–120.8 ms; FOV, 18 × 18 cm2; matrix, 256 × 256; 34 images; thickness, 3 mm; b = 0, 1000, 2500 s/mm2; bandwidth, 250 kHz.
PCT Measurements
PCT was performed on a 64-section V scanner (GE Healthcare). Eight adjacent 5-mm thick sections from a level just anterior to the optic chiasm to just anterior to the torcula were selected from a scout image. The CBV map was acquired after injection of iopamidol, 370 mg I/mL (Isovue 370; Bracco Diagnostics, Princeton, New Jersey), by using a dual-syringe power injector (Medtron, Saarbrücken, Germany). A monophasic injection of 12 mL of contrast (370 mg I/mL) at 1.5 mL/s was followed by a 12-mL saline chase at 1.5 mL/s. Following the power injection of the contrast medium and after a 5-second prep delay, a continuous scanning was initiated with the following parameters: 80 kVp, 200 mA, 1 second per rotation for 50 seconds. The 1-second images were reformatted at 0.5-second intervals.
Postprocessing was performed by an experienced technologist (K.P.) in conjunction with a senior neuroradiologist (C.M.S.) by using a commercially available CT perfusion software (GE Healthcare). For arterial and venous input functions, extracranial vascular structures were selected manually because of the small size of the canine intracranial arteries and the proximity of the superior sagittal sinus to the thick skull base.
C-Arm CT
Immediately following the PCT examination, C-arm CT was performed on a biplane flat-detector angiographic system (Artis dBA, Siemens Healthcare, Forchheim, Germany). The same contrast agent used in PCT (Isovue 370; Bracco Diagnostics) was injected into a peripheral vein with the same dual-syringe power injector (Medtron) used in the prior PCT examination.
Two CBV maps were acquired with the C-arm CT, by using 2 different injection protocols (described in detail below). To determine the time when there would be a steady level of contrast medium in the brain parenchyma, we injected contrast medium into a peripheral vein at the rate of 3.0 mL/s, and simultaneously with the start of the injection, a 2D-DSA acquisition was performed. From this, the time of full opacification of the superior sagittal sinus was noted. (At this time, contrast medium is present in arteries, parenchyma, and veins.) This time then was used to determine when normal parenchyma would have a steady level of contrast. To be sure that any parenchyma that might have slow flow as the result of perfusion through the collateral circulation also had a steady state of contrast filling during the acquisitions, we set the x-ray delay at 15–17 seconds for 1 acquisition and at 23–25 seconds for the second acquisition. We thought that this was prudent: Because we were not able to measure the dynamic elements of perfusion (MTT and CBF) with the C-arm technique, we were unable to determine if there was tissue downstream from an obstruction that, while having reduced MTT and CBF, was still maintaining normal or elevated CBV because of adequate but slow supply through collaterals. To be as sure as possible that we had allowed any such tissue to reach a steady state of enhancement, we extended the x-ray delay and performed the second CBV acquisition. After a 10-minute delay, the second CBV acquisitions were obtained. Each acquisition protocol consisted of 2 rotations: an initial rotation (mask run) followed by the power injection of the contrast medium, an appropriate x-ray delay, and then a second rotation (fill run).
Each of the 2 fill runs was started after injection of 25 mL of contrast agent at 1.0 mL/s for 25 seconds and at 1.5 mL/s for 17 seconds, respectively. Two hundred seventy-five projection images were obtained during a 10-second rotation in both instances. A 10-minute interval was taken between each acquisition.
In all animals except 1, the C-arm CT examinations were performed within a half hour of the PCT measurements (in 1 instance, the angiography suite was not available until 7 hours after creation of the stroke). All canines were euthanized after the final C-arm CT examination.
Data Analysis
PCT measurements were processed by using commercially available software (GE Healthcare). C-arm CT measurements were processed by using prototype software installed on a dedicated research work station (Leonardo, Siemens Healthcare). The reconstruction algorithm has been described previously.10 In brief, after reconstruction and subtraction of the mask run and the fill run separately, an algorithm is applied to further segment out air and bone from the image volume. The steady-state arterial input function value is then calculated from an automated histogram analysis of the vessel tree. A final scaling is then applied to account for the arterial input value and for other physiologic values (eg, hematocrit), before a smoothing filter is applied to reduce pixel noise.
An experienced researcher (K.P.) placed a region of interest manually on the PCT and C-arm CT CBV maps as close as possible to the center of the infarct as depicted on the DWIs. A symmetric region of interest was automatically prescribed in the contralateral normal hemisphere. Each region of interest measured 112 mm2 and contained both gray and white matter. The absolute values and the mean ratios (abnormal side to normal side) of these CBV values in the 2 regions of interest were calculated and compared.
Ten PCT CBV maps and 10 C-arm CT CBV maps from a previous study of normal canines were combined according to a computer-generated randomization scheme with the maps from the PCT and C-arm CT CBV studies of the 10 animals with ischemic stroke. The combined CBV maps (n = 50; 10 normal PCT, 10 normal C-arm CT, 10 stroke PCT, and 20 stroke C-arm CT) were then presented to 3 experienced observers (H.R., P.T., S.B.) who were blinded to the technique used to create the maps and to whether the maps were from normal or abnormal animals. The observers were asked to grade each map as being either normal or abnormal and, if abnormal, to designate the side of the abnormality.
A 2-sample paired t test was used to compare the mean values of the region of interest within the infarcted territory and the corresponding normal tissue of the PCT and the 2 C-arm CT injection protocols. Descriptive statistics were performed for assessment of the 3 readers' performances in identifying the infarcts.
Results
No complications occurred during creation of the strokes. DWI identified cerebral infarcts in 9 of the 10 canines. In 1 canine with a large area of ischemia on the DWI, there was a 3-hour delay between performing the PCT and C-arm CT due to unavailability of the C-arm CT equipment. In this canine, C-arm CT was performed >7 hours after creation of the embolic stroke and there was no evidence of intracranial circulation at the time that the C-arm CT was performed.
Significant differences in the CBV values between the infarcted territory and the normal brain were found in both the PCT CBV maps and the C-arm CT CBV maps acquired with both injection protocols in the 8 animals with proved ischemic strokes (P < .005). Conversely, no significant differences between the CBV measurements obtained on PCT and C-arm CT with either injection protocol in both the infarcted and normal territories were detected (P > .05) (Figs 1 and 2). The results of CBV measurements in all imaging modalities are summarized in Tables 1 and 2. Because we did not have a means to match (register) exactly the CBV maps from the PCT and the C-arm CT studies, it is likely that there was some difference in their locations.
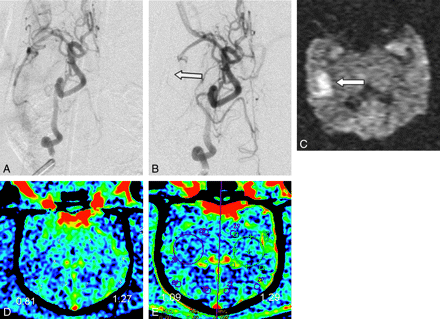
A−C, DSA with selective ICA injection pre- (A) and postembolization (B) at the origin of the ICA, resulting in an occlusion of the right MCA (arrow in B). C, DWI performed 4 hours later confirms the presence of a right MCA infarct (arrow). D, C-arm CT and PCT (E) demonstrate corresponding decreased CBV as determined both by the color maps and measured values in the respective regions of interest. CBV values are in milliliters per 100 grams of brain tissue.
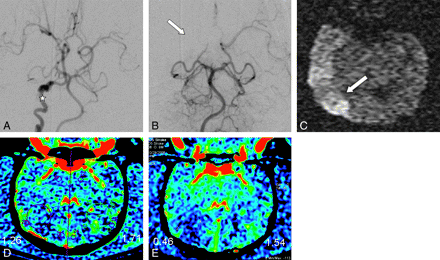
A and B, DSA with selective ICA injection pre-embolization demonstrates tortuosity of the ICA (asterisk in A). Embolization was performed near the origin of the right MCA, from a vertebral artery approach leading to occlusion of the proximal MCA. MCA infarct (arrow) was validated by DWI (C) done 4 hours after embolization. C-arm CT (D) and PCT (E) reveal corresponding decreased CBV as determined both by the color maps and measured values in the respective regions of interest.
Mean CBV values in regions of interesta
Mean CBV ratios (infarct hemisphere/normal hemisphere) in regions of interesta
The 3 expert readers made the correct diagnosis in 73.3% of the maps on the basis of PCT acquisitions and in 84.4% of the maps on the basis of C-arm CT acquisitions. They came to false-positive results in 1.7% and 3.3% of maps on the basis of PCT and C-arm CT images, respectively. False-negative evaluations of PCT and C-arm CT maps occurred in 25% and 12.2% of cases, respectively. The results of the evaluation of the CBV maps derived from the 2 different C-arm CT injection protocols did not differ significantly. The individual performances of the 3 readers' image evaluations are shown in Table 3.
Readers' performance in evaluating PCT and C-arm CT imagesa
Discussion
This study has shown that measurement of CBV in canines with an acute stroke is feasible with C-arm CT and that the results are comparable with those acquired with standard PCT.
A key benefit of this technique lies in the potential for measurements of CBV acquired during an intervention in the angiography suite. As improvements in the image quality of C-arm CT occur, this then would provide the potential to triage, treat, and monitor subjects with acute ischemic stroke in the angiography suite (an initial noncontrast C-arm CT would be used to exclude hemorrhage, etc, and a CBV measurement would be used to distinguish ischemic core from penumbra). Sequential measurements could be used both to assess the effects of treatment and to provide ongoing feedback as to tissue viability during long interventions. The value of CBV in differentiating regions of ischemic white matter that are salvageable from regions of ischemic white matter that are infarcted has recently been demonstrated.11
Today, C-arm CT does not provide image quality that is adequate for excluding a small intracranial hemorrhage. For this purpose, a standard noncontrast-enhanced CT or MR imaging is required.12,13 In a tertiary care center, however, patients often present with existing CT scans from outside institutions that have excluded hemorrhage before their referral. In such instances, CT or MR imaging examinations are usually ordered to assess the physiologic state of the brain before making a decision as to whether to attempt revascularization. Subjects in this category could potentially benefit from being sent directly to the angiography suite where physiologic triage could be achieved by using CBV maps obtained with C-arm CT. Ongoing improvements that reduce scatter radiation, increase temporal resolution, and correct for motion artifacts have the real potential to improve the image quality of C-arm CT to a level in which both initial assessment and triage of patients with an apparent acute ischemic stroke are feasible in the angiographic suite.14–16
As we discussed in our article describing the measurement of CBV with C-arm CT in normal canines, in situations in which an assessment of CBV during a therapeutic intervention would be helpful, it may be an advantage to inject contrast medium intra-arterially (aortic arch, ICA, VA) to make regional measurements of CBV.10 This would allow a significant reduction in the amount of contrast medium required and would, thus, enhance the ability to perform sequential measurements during a therapeutic procedure. We are currently exploring further the potential benefits and limitations of intra-arterial injections by using our canine model.
The technique used to create strokes in our study was based on earlier reports.17,18 In hopes of creating a high incidence of strokes in these animals and also hoping to create strokes of varying sizes, we modified the described technique by using combinations of autologous clot, silk suture, and Avitene as embolic materials. We also adapted our route to access the MCA territory according to the specific anatomy of each animal because in some canines, the MCA territory was not approachable via the ICA due to its tortuosity.17 In those instances, we reached the MCA territory via the vertebrobasilar route.
There are several limitations to our study. The image quality of all of the CBV maps obtained in this study was suboptimal compared with those obtainable in human subjects. This was primarily the result of a variety of factors: a very thick skull surrounding a very small brain and the application of software algorithms optimized for human subjects rather than for canines. Because of our inability to match (register) exactly the CBV maps from the 2 modalities, one must assume that there were some minor differences in their locations. Such variation would then be reflected in the absolute and relative measurements of CBV within the regions of interest. Because these values have been demonstrated to be useful in distinguishing ischemic core from penumbra, it is important that, in future studies, CBV maps from C-arm CT be registered accurately from maps made with other modalities.19,20
The small dose of contrast medium used for the PCT studies combined with the interval between the subsequent study with C-arm CT make it unlikely that there was significant residual circulating contrast from the PCT study at the time that the C-arm CT acquisitions were performed. Additionally, the arterial input function used in the C-arm measurements is based on the Hounsfield unit value of “pure” blood as measured in the subtracted volume. This would mean that any residual contrast should be subtracted and, thus even if present, should not impact the measurements. If, however, there was, for any reason, “unsubtracted” residual contrast, the impact on lower CBV values could be relatively higher than that on higher CBV values.
Conclusions
Measurements of CBV with C-arm CT compared well with measurements made with PCT in an acute stroke canine model. The ability to measure CBV in the angiographic suite in subjects with acute ischemic stroke would enhance the environment in which revascularization procedures are performed.
Acknowledgments
We thank our colleagues, Drs Patrick Turski, Howard Rowley, and Søren Bakke, who reviewed and scored the CBV maps.
Footnotes
This work was supported by Siemens Healthcare, Forchheim, Germany.
References
- Received April 15, 2009.
- Accepted after revision July 27, 2009.
- Copyright © American Society of Neuroradiology