Abstract
BACKGROUND AND PURPOSE: If blood flow in the ICA is reduced by the use of a distal filter during CAS, flow stagnation proximal to the filter occurs and this increases the probability of floating debris. The floating debris that remains after filter retrieval may cause cerebral embolism. However, if blood flow is increased by aspiration of blood from the ICA through an aspiration catheter, debris could be removed while the filter is still in place. The purpose of this study was to investigate blood flow changes in the ICA induced by filter use and aspiration.
MATERIAL AND METHODS: A filter-protection device (AngioGuard XP) was used during CAS in 13 consecutive patients with carotid stenosis. Blood flow velocity in the ICA was measured by carotid Doppler sonography during filter deployment, filter retrieval, and catheter aspiration.
RESULTS: Blood flow velocity significantly decreased with filter placement and significantly increased with filter retrieval in patients with normal angiographic flow (P < .05). Aspiration of a 20-mL blood sample from the proximal ICA column significantly increased the blood flow velocity (P < .05).
CONCLUSIONS: The blood flow changes in the ICA induced by the use of a distal filter may cause cerebral embolism in filter-protected CAS. A routine aspiration method can potentially reduce the amount of migrating debris during CAS, even in cases with angiographic normal flow.
Abbreviations
- AG
- AngioGuard
- ANOVA
- analysis of variance
- CAS
- carotid artery stenting
- DSA
- digital subtraction angiography
- EDV
- end-diastolic velocity
- ICA
- internal carotid artery
- NASCET
- North American Symptomatic Carotid Endarterectomy Trial
- Post
- after
- Pre
- before
- PSV
- peak systolic velocity
- TAMV
- time-averaged maximum velocity
In CAS, distal filter protection devices may potentially reduce the incidence of intracranial embolization, which may be caused by debris liberated from atheromatous plaques. However, embolic complications are still a major problem in filter-protected CAS.1–4 When the antegrade flow of contrast medium through the ICA is obstructed or markedly reduced on DSA before retrieval of the filter, blood aspiration from the ICA column through an aspiration catheter is recommended before filter retrieval.5 Blood flow impairment is thought to create stagnation of blood flow just proximal to the filter, which may cause floating debris in the proximal ICA column. In contrast, when DSA shows no blood flow impairment in the ICA, the filter device is retrieved without aspiration because the debris is assumed to have been captured in the filter basket.
Sorimachi et al6 reported that regardless of the flow condition of contrast medium on DSA, a substantial amount of debris was always collected in aspirated blood during CAS with distal filter protection. There are several hypothetic mechanisms that might explain effective debris capture by using the routine aspiration method. Even when slow flow is not evident angiographically, blood flow in the ICA is partially disturbed by the filter as shown in experimental studies by using a carotid bifurcation model.7,8 This blood flow disturbance creates stagnation of blood flow proximal to the filter, which may cause floating debris in the proximal ICA. After closure of the filter basket, increased blood flow in the ICA results in a proportional increase in wall shear stress, which liberates debris from vulnerable plaque that has been compressed by the balloon and stent.
Migration of both liberated debris and floating debris into the cerebral arteries may cause cerebral embolism. With the aspiration method, however, pressurized blood flow through the ICA column induced by the negative pressure of a vacuum syringe through an aspiration catheter lumen leads to increased ICA blood flow while the filter is in place. The blood flow increase facilitates the release of vulnerable fragments from plaque through the stent struts. Removal of both floating debris and released debris from the ICA through the aspiration catheter may prevent cerebral embolism after the filter is retrieved.6
To the best of our knowledge, blood flow changes before and after filter deployment and retrieval and before and during aspiration have never been reported in the clinical setting. Thus, the purpose of this study was to use carotid Doppler sonography to measure blood flow changes in the ICA induced by filter devices and aspiration catheters that are used during CAS. The blood flow changes that we observed support the hypothetic mechanisms described above; thus, our findings may explain the effectiveness of the routine aspiration method in the prevention of cerebral embolism.6
Materials and Methods
Patient and Lesion Characteristics
This study was conducted from September 2009 to May 2010, in 13 consecutive patients (mean age, 69.9 years; range, 61–80 years; 13 men) with lesions that were treated with CAS with distal filter protection at the University of Niigata. No women underwent CAS during the study period. Informed consent was obtained from all patients. The study protocol was approved by the institutional review board of the University of Niigata. The degree of carotid artery stenosis was calculated on the basis of the NASCET criteria.9 Indications for CAS included an asymptomatic carotid artery stenosis of at least 80% or a symptomatic carotid artery stenosis of at least 50%. Patients were excluded from CAS if they had a previously disabling stroke. Six lesions were symptomatic and 7 lesions were asymptomatic. Carotid artery stenosis was located on the right in 7 lesions and on the left in 6. Stenoses of the carotid artery varied from 60% to 95%, with an average of 78.8%.
Carotid Stent-Placement Procedure
Antiplatelet therapy consisting of orally administered aspirin (100 mg) and clopidogrel (75 mg) or cilostazol (200 mg) was started at least 5 days before the procedure. During the procedure, heparin was given by intravenous infusion to maintain the activated clotting time at >2 times baseline, with a target of at least 300 seconds. Percutaneous access was gained through the femoral artery. Guiding catheters (8F) were advanced into the common carotid artery. Stenoses were crossed with a filter-protection device (AngioGuard XP; Cordis, Miami Lakes, Florida). The filters had diameters of 5, 6, and 7 mm; a filter 0.5–1.5 mm larger than the distal vessel diameter measured by DSA was chosen. After opening the filter, we performed intravascular sonography to evaluate the diameter of the stenotic lesion and the plaque characteristics. Predilation with 3.5- to 4.0-mm-diameter angioplasty balloons was performed at 6 or 8 atm. Self-expanding stents (Precise, Cordis) were implanted covering the carotid bifurcation. A stent 1–2 mm larger than the proximal common carotid artery diameter was selected. When the immediate poststent residual stenosis was approximately >33%, the stent was postdilated with 4.5- to 6.0-mm-diameter balloons at 10 atm. After postdilation, a routine aspiration method was performed regardless of the angiographic flow state.6 An Eliminate 7F aspiration catheter (lumen diameter of the distal tip was 1.25 mm; Clinical Supply, Gifu, Japan) with a 30-mL proximal vacuum syringe was advanced toward the filter. The tip of the aspiration catheter was located just proximal to the AngioGuard XP. After aspiration of approximately 20 mL of blood, the aspirated blood was passed through a Falcon cell strainer with a pore diameter of 40 μm (BD Biosciences, Bedford, Massachusetts). Visual inspection of the cell strainer was performed by the operator to evaluate the macroscopic debris present. Aspiration of approximately 20 mL of blood was performed at least 5 times, and then the aspiration was continued until almost no debris was observed macroscopically in the cell strainer. Finally, a retrieval sheath was advanced, and the filter was closed and removed from the artery. The repeated blood aspirations took approximately 3–5 minutes from postdilation to the removal of the filter.
Flow Assessment on DSA
Angiography was systematically performed to assess flow after each of the following steps of the procedure: filter device placement, balloon predilation, stent placement, balloon postdilation, aspiration of the blood column, and filter-device retrieval. Ioxaglic acid (320 mg) was used as the contrast medium and was injected by hand. DSA images (3 frames/s) were obtained in a lateral view with a vertical field of approximately 20 cm. During CAS procedures, the operators classified blood flow immediately before filter retrieval as normal flow, slow flow, or stop flow. “Stop flow” was defined as antegrade flow cessation. “Slow flow” was defined as new and definite flow impairment compared with the flow in the external carotid artery.10 The CAS operators for each procedure were 2 of the 4 authors (T.S., K.N., Y.I., K.M.) and were board-certified neurointerventionalists and neurosurgeons with >15 years' experience in their specialties. In addition to prospective data collection on the incidence of flow impairment, experienced interventionists reviewed all angiograms to qualitatively assess flow in the ICA.
Carotid Doppler Assessment
Carotid Doppler sonography was systematically performed to assess flow during the following steps of the procedure: filter-device placement, aspiration of the blood column, and filter-device retrieval. To evaluate pulse-wave changes induced by deployment of the AngioGuard XP, we started the pulsed-wave Doppler recording after the AngioGuard XP system was placed at the target site. Then, the deployment sheath was removed, and the filter was deployed inside the arterial lumen. The recording was continued for at least 10 seconds after filter deployment. To measure flow changes induced by aspiration, we started the pulsed-wave Doppler recording after the aspiration catheter was advanced to a position just proximal to the basket, and then we performed aspiration. The pulse wave was continuously recorded for at least 10 seconds after completion of the aspiration. For measurement of flow-velocity changes caused by retrieval of the AngioGuard XP, the capture sheath was advanced to a position just before the filter basket, and the pulsed-wave Doppler recording was started. Then, the basket was captured by using the capture sheath, and the system was removed. The pulse wave was continuously recorded until the entire system was retrieved into the guiding catheter.
During the recording periods with cooperation from the patients, we tried to avoid motion artifacts, including swallowing. A carotid Doppler examination measured PSV, EDV, and TAMV. All examinations were performed by the same examiner (T.S.) by using the same sonography machine (Sonovista X300; Mochida Siemens Medical Systems, Tokyo, Japan). The B-mode imaging frequency was 8 MHz, and the pulsed-wave Doppler frequency was 5.3 MHz. Velocity in the ICA was measured within 3 cm of the bifurcation. We attempted to maintain an insonation of 60° at all times, if possible, to standardize the study methodology.
Debris Particle Analysis
The aspirated debris in the 40-μm mesh cell strainer was rinsed with saline and immersed in 50 mL of 10% neutral buffered formalin with the cell strainer for fixation. Particles floating in the 50 mL of formalin were stained with 0.04 mL of 1% Pyoktanin blue solution for visualization of the debris and filtered through the cell strainer.6 The particles on the membrane of the cell strainer were subsequently counted under a stereoscopic microscope by 2 authors (T.S. and K.N.). The maximum diameter of the particles was measured, and the particles were divided into 3 groups based on diameter: Group 1 had a maximum diameter of ≥1 mm, group 2 had a maximum diameter of <1 but ≥0.5 mm, and group 3 had a maximum diameter of <0.5 but ≥0.1 mm. The mean number of debris particles determined by the 2 authors was defined as the final particle number.
Statistical Analysis
Statistical evaluation was performed by using the paired t test for comparisons of ICA blood flow velocity before and after filter placement and filter retrieval, and before and during aspiration. A repeated-measures ANOVA was performed to compare the number of debris particles in all of the aspirated blood samples. If statistically significant differences were observed by ANOVA, then a Dunnett test was used to compare the first blood sample with the subsequent samples. A value of P < .05 was considered significant. All statistical analyses were performed with StatView 5.0 (SAS Institute, Cary, North Carolina).
Results
Clinical Results
In all 13 CAS procedures, the carotid stent was placed correctly. All stents were postdilated. After CAS, the ICA had a residual stenosis of <30% in all cases as assessed by NASCET criteria. A complication associated with the CAS occurred in 1 patient. This patient had an intracerebral hemorrhage caused by hyperperfusion syndrome with a minor visual field defect 2 days after the procedure. He returned to his previous work 2 months after the procedure.
Blood Flow Changes Induced by Deployment of the AngioGuard XP
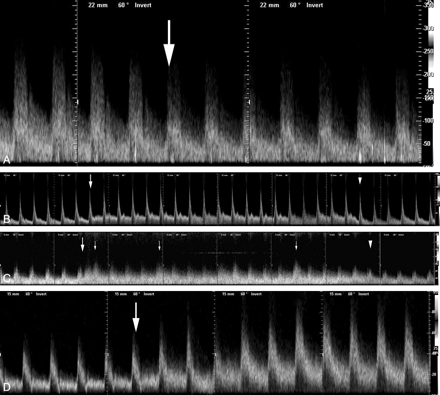
In all 13 patients, blood flow impairment was not demonstrated on DSA after deployment of the AngioGuard XP. Figure 1A shows a pulse wave recorded during deployment of the AngioGuard XP in a representative case. Table 1 indicates blood flow changes induced by filter deployment. In 5 patients, blood flow velocity could not be measured because of technical problems, including motion artifacts associated with swallowing. In the remaining 8 patients, PSV, EDV, and TAMV significantly decreased after filter deployment compared with the velocities measured before filter deployment (P < .05). In these 8 patients, the PSV, EDV, and TAMV decreased after filter deployment by mean values of 27.9%, 32.3%, and 22.9%, respectively.
Pulse waves of the ICA during a CAS procedure. A, Blood flow change induced by deployment of an AngioGuard XP in a 73-year-old man with 90% ICA stenosis. Opening the filter basket reduces the blood flow velocity (arrow). B, Blood flow change induced by aspiration in a 67-year-old man with 70% ICA stenosis after postdilation. The blood flow velocity increases immediately after beginning aspiration (arrow) and decreases after finishing aspiration (arrowhead). C, Blood flow change induced by aspiration in a 65-year-old man with 90% ICA stenosis after postdilation. The blood flow velocity increases immediately after beginning aspiration (large arrow) and decreases after finishing aspiration (arrowhead). Amplitudes of some pulses (small arrows) are larger than others. D, Blood flow change induced by retrieval of an AngioGuard XP in a 61-year-old man with 60% stenosis after aspiration following postdilation. Closure of the filter basket increases the blood flow velocity (arrow).
Changes in blood flow velocity in the ICA during carotid stenting
Blood Flow Changes Induced by Catheter Aspiration
Aspiration through an aspiration catheter was performed ≥5 times in each CAS procedure; then during aspiration, ICA pulse waves were recorded several times in each patient. The aspiration showing the largest change in PSV was used for evaluation in this study. Figures 1B and C demonstrate blood flow changes in pulsed-wave Doppler sonography before, during, and after aspiration. The amplitude of blood flow increase fluctuated during each aspiration. Table 1 indicates blood flow changes induced by catheter aspiration. In 1 patient, the flow velocity could not be measured because of a technical problem. During aspiration, PSV, EDV, and TAMV increased significantly compared with before aspiration (P < .05). The mean increases of blood flow velocity induced by aspiration were 50.1% in PSV, 63.7% in EDV, and 45.5% in TAMV.
Blood Flow Changes Induced by Retrieval of the AngioGuard XP
Before retrieval of the AngioGuard XP and after several aspirations following postdilation, DSA showed normal flow in 12 patients and slow flow in 1 patient. Figure 1D shows the pulse-wave change recorded in a subject with normal flow during closure of the filter. The case with slow flow on DSA was excluded because the ICA could not be identified by B-mode imaging before filter retrieval. Table 1 indicates changes in blood flow before and after filter retrieval in the 12 patients with normal flow. After retrieval of the filter, there were significant increases in PSV, EDV, and TAMV (65.6%, 78.4%, and 71.9%, respectively; P < .05).
Microscopic Analysis of Captured Debris
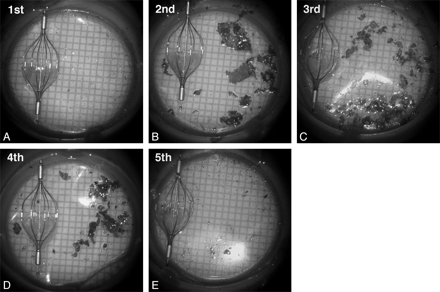
In all 13 CAS procedures, a substantial amount of debris was macroscopically found in the cell strainer that was used to filter the aspirated blood (Fig 2). Debris particles with a diameter of ≥1 mm were found in all 13 procedures. The number of debris particles ≥1 mm was 1–9 in 3 procedures (23.1%), 10–19 in 5 procedures (38.5%), 20–29 in 2 procedures (15.4%), 30–39 in 2 procedures (15.4%), and ≥40 in 1 procedure (7.7%). Table 2 shows the relationship between the order of blood-aspiration samples taken and number of debris particles in the aspirated blood samples. The number of debris particles with a maximum diameter of ≥1 mm was significantly larger in the second, third, and fourth aspirated blood samples compared with the first aspirated sample (P < .05). The difference was not significant between the first aspirated sample and the fifth aspirated sample. The number of debris particles with a maximum diameter <1 but ≥0.5 mm was significantly larger in the second, third, fourth, and fifth aspirated blood samples compared with the first aspirated sample (P < .05). The number of debris particles with a maximum diameter <0.5 but ≥0.1 mm was not significantly different when comparing the first aspirated sample with each of the other aspirated samples.
Photographs of debris captured in cell strainers from the first-through-fifth aspirations of blood from the ICA column during 1 procedure. The debris in the cell strainer is shown beside a 6-mm-diameter AngioGuard XP for comparison. Larger amounts of debris are captured in the second, third, and fourth 20-mL aspiration samples compared with the first sample. The debris, stained with Pyoktanin blue solution, is filtered through the cell strainer.
The number of debris particles in the first aspirated blood sample compared with each of the other aspirated blood samples
Discussion
The present study demonstrated a significant decrease in blood flow velocity in the ICA with placement of the AngioGuard XP and a significant increase with its retrieval. A mean decrease of 27.1% in PSV was induced by filter placement, and a mean increase of 65.6% in PSV was observed after filter retrieval. In an in vitro study reported by Hendriks et al,7 a decrease of approximately 10% in blood flow was observed with AngioGuard XP placement in an artificial carotid bifurcation model. Siewiorek et al8 reported a 2% blood flow reduction caused by placing an AngioGuard XP in a carotid bifurcation model with ICA stenosis and a 5% reduction caused by an AngioGuard XP filled with 200-μm-diameter polymer microspheres. Compared with these in vitro study results, the present in vivo study showed a larger reduction in the rate of blood flow with AngioGuard XP deployment. There are several mechanisms that may account for the difference in flow-reduction rates between the in vivo and in vitro studies. A silicone carotid bifurcation model does not exhibit the same soft-tissue mechanics as a human carotid artery, including gaps between the vessel wall and the basket rim of the device. Moreover, endothelial damage and spasms due to device deployment cannot be observed in synthetic flow models. The use of constant flow rates instead of pulsatile flows in these 2 studies by using carotid models limits the performance of the filter devices under physiologic conditions. In vitro studies usually use polymer microspheres as artificial debris particles,8,11,12 though the shape and texture of real debris particles, which are flat and soft,6 are different from those of polymer microspheres.
In the present study, blood flow reduction caused by filter-device placement was always shown by carotid Doppler sonography when impairment of contrast medium flow was not apparent on DSA. In 12 patients with DSA findings of normal flow before retrieval of the filters, a significant increase in PSV was observed immediately after filter retrieval. When the mean flow velocity was 40 cm/s, the blood stream advanced approximately 13 cm for each DSA frame; a frame rate of 3 frames/s was used in the present study. The tip of the contrast medium in the ICA could be observed in only 1 or 2 frames on DSA with view fields of 20–30 cm. PSV in a human ICA is usually >3 times the EDV; therefore, the timing of the contrast medium injection during the cardiac cycle affects the flow speed of the tip of the contrast medium. For these reasons, DSA is difficult to use for evaluation of blood flow reduction in the ICA during CAS, unless a severe blood flow reduction occurs. Especially during filter deployment, accelerated blood flow through the stenotic ICA prevents detection of blood flow changes by means of DSA at 3 frames/s.
The present carotid Doppler study demonstrated that the use of a distal filter device always reduced blood flow in the ICA. This blood flow reduction creates stagnation and a vortex in the blood flow just proximal to the filter basket, which may cause floating debris in the ICA proximal to the filter. After closure of the filter basket, an increase in blood flow causes a proportional increase in wall shear stress in the ICA, which probably releases fragments from vulnerable plaque and debris particles trapped between the stent struts and the arterial wall. This process is one of the mechanisms suspected of causing cerebral embolism after filter retrieval.
While the filter is in place, a blood flow increase in the ICA is induced by the negative pressure of a vacuum syringe through an aspiration catheter. In the present study, a mean increase of 50.1% in PSV was observed during catheter aspiration. With the routine aspiration method reported by Sorimachi et al,6 aspiration of 20 mL of blood into a syringe took approximately 10–15 seconds; therefore, the blood-aspiration rate was approximately 1.3–2 mL/s. This blood-aspiration rate is approximately half of the normal blood flow rate in the ICA, which is usually 3–4 mL/s. This additional blood flow induced by the blood aspiration could cause an increase in blood flow velocity in the ICA. Potential mechanisms for the amplitude fluctuation of the blood flow velocity observed in the present study include temporary disruption in the laminar flow pattern in the ICA by blood aspiration and partial obstruction of the aspiration catheter lumen by aspirated debris particles. The blood flow increase caused by aspiration probably facilitates the release of vulnerable fragments from plaque through the stent struts during filter use. The volume of the ICA column proximal to the filter basket is <10 mL; therefore, most of the debris particles that are already floating before aspiration should be captured in the first aspiration of 20 mL of blood. Sorimachi et al6 reported that a significantly larger number of debris particles ≥1 mm were captured in the second, third, and fourth aspiration samples (20 mL) than in the first aspiration sample. This finding is consistent with the results of the present study and suggests that a larger proportion of debris particles ≥1 mm in diameter are liberated by the aspiration method itself compared with particles floating before aspiration.
Limitations
Blood flow velocities measured in the same artery by using carotid Doppler sonography are not consistent under different conditions, depending on the position and angle of the Doppler probe to the artery. In the present study, pulse waves were continuously recorded during deployment and retrieval of filters and aspiration. Therefore, a comparison of pulse-wave amplitudes is possible for each recording, though blood flow velocity values cannot be compared between different recordings.
Conclusions
The present carotid Doppler study demonstrated that blood flow velocity significantly decreased with filter-device placement and significantly increased with filter-device retrieval in all patients undergoing CAS, including patients without apparent angiographic flow impairment of contrast medium (P < .05). These blood flow changes are speculated to cause cerebral embolism during CAS by using distal filters. Aspiration of blood samples in the proximal ICA column by using an aspiration catheter significantly increased blood flow velocity in the ICA during filter use (P < .05). The routine aspiration method, in which several blood samples are aspirated, is thought to reduce the amount of migrating debris during CAS, even in cases with normal angiographic flow.
References
- Received May 21, 2010.
- Accepted after revision July 3, 2010.
- Copyright © American Society of Neuroradiology