Abstract
SUMMARY: Venous malformations have previously been reported to be associated with skeletal changes in humans, typically demineralization and localized deformation of the long bones. We report a presumed developmental association between venous malformations of the head and neck and meningoencephaloceles involving the sphenoid and temporal bones. Recognition of this association is important to avoid misinterpretation of these changes as a more aggressive process. We present the imaging findings and suggest an embryologic basis for this previously unreported association.
In 1982, Mulliken and Glowacki1 proposed a classification scheme for vascular malformations that forms the cornerstone of current understanding of vascular anomalies. Venous malformations are low-flow lesions that have characteristic imaging features.2 Previous studies have demonstrated that venous malformations may be associated with changes in adjacent skeletal structures, including deformity, erosion, hypoplasia, and hypertrophy.3 These changes, the result of local mechanical effects, are generally considered to be acquired.
We report the CT and MR imaging findings of 3 patients with ipsilateral meningoencephaloceles and venous malformations of the head and neck in whom the bony changes do not appear to be exclusively a result of localized mechanical or pressure effects. While the precise nature of this previously unreported association is not fully understood, we propose an embryologic rationale for its occurrence.
Case Reports
Case 1
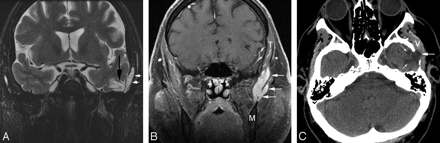
A 66-year-old man with no significant medical history presented with left-sided facial discomfort and headache associated with cough. An MR image was interpreted as showing a skull base mass, and the patient was referred to our institution for resection of a presumed tumor. An MR image of the skull base performed at our institution (Fig 1) revealed a defect in the left inferior temporal squamosa associated with protrusion of gliotic brain and meninges, consistent with a meningoencephalocele. In addition, a well-circumscribed lobulated soft-tissue mass was present in the masticator space, extending along the temporalis muscle from the ramus of the mandible to the suprazygomatic region and partly in contact with the temporal bone. Its imaging characteristics were strongly suggestive of a venous malformation, but confirmatory testing was requested by the referring physician to ensure that a potentially surgical (ie, neoplastic) lesion was not present. Because the lesion was difficult to distinguish from muscle on CT, an MR imaging−guided needle biopsy was performed, which yielded only blood from the center of the lesion, with no evidence of malignancy. Iodinated contrast material was then injected into the lesion, with subsequent CT evaluation demonstrating irregular central contrast accumulation consistent with a venous malformation. These lesions have remained stable for 5 years without treatment.
A, Coronal fast spin-echo T2-weighted image with fat suppression demonstrates herniation of gliotic brain parenchyma and meninges (black arrow) through a defect located in the left inferior temporal squamosa at the sphenotemporal junction. An adjacent ovoid T2 hyperintense structure (white arrows) was separate from the meningoencephalocele and represents a portion of a large left facial venous malformation. B, A more anterior coronal postgadolinium T1-weighted image with fat suppression demonstrates a homogeneously enhancing lobulated mass consistent with a venous malformation (white arrows), which extends from the suprazygomatic masticator space above (temporalis muscle [T]) to the mandible (M) below. C, Axial CT image demonstrates a bony defect in the inferior aspect of the temporal squamousa, with gliotic brain (small white arrows) and herniation of the meninges and subarachnoid space laterally (large white arrow). The adjacent venous malformation (V) is also indicated.
Case 2
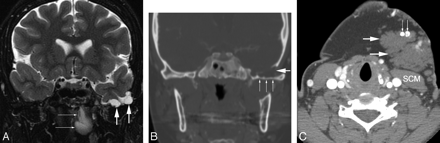
A 26-year-old woman was referred for evaluation of severe bifrontal headaches. The patient's medical history was significant for a left submandibular venous malformation previously treated with percutaneous sclerotherapy and also for left chronic otitis media with tympanic membrane perforation, previously treated with tympanoplasty and canaloplasty. MR imaging performed for further assessment of the patient's headache revealed that the left greater wing of the sphenoid was thinned, scalloped, and focally dehiscent. The left temporal lobe was seen to herniate inferiorly, with some distortion of the gyri but no clear areas of encephalomalacia (Fig 2). A large T2 hyperintense lesion consistent with a venous malformation was seen to involve the left carotid, pharyngeal mucosal, buccal, and submandibular spaces, abutting the undersurface of the sphenoid bone. A noncontrast CT scan demonstrated phleboliths within this lesion, confirming venous malformation. This patient's condition and her scans have remained stable during 5 years of follow-up.
A, Coronal fast spin-echo T2-weighted image with fat saturation demonstrates inferior herniation of the left temporal lobe and subarachnoid space (short thick arrows), with distortion of the left temporal lobe but no frank gliosis. In addition, a portion of the patient's venous malformation is seen involving the nasopharynx (thin white arrows). B, Coronal CT image at a level similar to that in A reveals multifocal thinning of bone along the left greater wing of the sphenoid (small white arrows), as well as a focal defect in the inferior temporal bone (large white arrow). C, An axial CT image at the level of the upper neck shows additional areas of venous malformation (large white arrows), anterior to the sternocleidomastoid muscle (SCM) and containing phleboliths (small white arrows).
Case 3
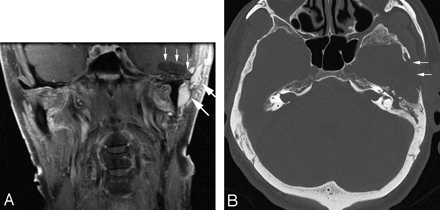
A 75-year-old man presented with left cheek fullness, darkening of the left pinna for the past 5 years, and worsening left pulsatile tinnitus. His medical history was notable for a left parotid region venous malformation, previously treated with parotidectomy and subtotal resection. MR imaging and CT demonstrated a left-sided venous malformation involving the masticator and residual parotid spaces, as well as the external auditory canal; a discontinuous region of venous malformation involved the superomedial aspect of the left orbit. There were localized areas of bony deficiency involving the petrous bone and middle ear. Additional bony deficiency with focal meningoencephalocele formation was present at the level of the greater wing of the sphenoid and the left temporomandibular joint (Fig 3). This patient's condition has also remained stable for a more limited follow-up of 1 year.
A, Coronal T1 postgadolinium image with fat saturation demonstrates cystic encephalomalacia of the left temporal lobe with a focal cephalocele (thin vertical arrows), as well as lobulated enhancing masses (large white arrows) consistent with a residual/recurrent venous malformation. B, An axial CT image in a bone window demonstrates loss of bone involving the left temporal squamosa (white arrows).
Discussion
We present 3 cases in which a developmental vascular anomaly, notably a venous malformation, is associated with an abnormality of the skull base. We suggest that the occurrence of these lesions in the same patient has a developmental basis rather than simply representing either a chance association or localized pressure effects of the venous malformation on bone.
A number of prior reports comment on an association between vascular malformations and changes in adjacent bone. Vascular malformations have been associated with bony distortion, destruction, hypertrophy, hypoplasia, attenuation changes, and primary intraosseous involvement. Other than in situations of primary intraosseous involvement, these changes have been considered to result from localized mechanical forces or flow effects. In 1 study, 34% of vascular malformations of the head and neck demonstrated some form of bony alteration.4 Venous malformations, a subcategory of vascular malformations, have been described as causing hypoplasia, demineralization, and osteolysis of long bones. These bony changes are generally attributed to alterations in local temperature, local oxygen content, and venous flow.5–8 Venous malformations, via venous hypertension and stasis, can also induce bony hypertrophy through an organized periosteal reaction that leads to cortical thickening.3,4,9
Certain rare syndromes are known for bony changes in association with vascular malformations. These include Klippel-Trenaunay syndrome, Servelle-Martorell angiodysplasia, Maffucci syndrome, and Gorham-Stout syndrome.10–15 None of these syndromes with vascular and osseous changes are consistent with the findings in our otherwise healthy patients.
In our patients, the skull base abnormality is not clearly a direct result of the venous malformation. Though the skull defect and the venous malformation are ipsilateral and in close proximity, especially in patient 1, they are not in direct contact over the full extent of the bony abnormality. Instead, these appear to be associated developmental anomalies. We reviewed the development of the sphenoid wing and temporal squamosa as well as the development of venous anomalies of the head and neck in search of possible mechanisms of co-occurrence.
There is evidence for an association of skull anomalies with altered development of venous structures. A number of studies have reported the association of atretic and nonatretic cephaloceles (typically occipital and parietal) with abnormal venous drainage patterns.16–19 These studies emphasize the timing of development of the osseous structures and venous sinuses. Otsubo et al 16 discuss the possibility that if a parietal cephalocele existed as a result of a neural tube defect, the left and right primitive marginal sinuses would not be able to fuse together at the midline around day 50 of embryonic development to form the superior sagittal sinus; hence, the superior sagittal sinus would appear fenestrated, as it did in the cases they describe. These studies highlight an association between cephaloceles and altered venous drainage patterns, however, and not an association with venous malformations.
A recent study in the ophthalmology literature established a previously unrecognized association between orbital varices, cranial defects, and encephaloceles. Orbital varices are defined as a plexus of low-pressure, low-flow, thin-walled, and distensible vessels that can communicate with normal orbital veins.20 Three main types of anomalies are reported in association with orbital varices: large midline cranionasal deformities, large superomedial defects of the orbital wall, and defects involving the greater wing of the sphenoid. The study suggests that the geographic proximity of the orbital varix to the cranial bone anomaly may be due to a “local failure” during craniofacial development and suggests that these lesions are causally related.
Venous malformations can be considered to be localized errors of angiogenic development.21 Vascular morphogenesis begins relatively early, at approximately 13–15 days gestation. This process is divided into 2 phases: vasculogenesis and angiogenesis. Vasculogenesis begins in the extraembryonic mesoderm of the yolk sac where mesodermal hemangioblasts congregate into clusters of blood islands.22 Angiogenesis describes the physiologic process whereby new blood vessels sprout and grow from pre-existing blood vessels. While the precise developmental mechanism of venous malformation is not understood, an error in angiogenic development leading to the formation of a venous malformation can occur as early as 2 weeks' gestation. In contrast, the earliest evidence of skull formation is during week 4 of gestation, when paraxial mesoderm and neural crest cells migrate to form the base of the ectomeningeal capsule.23
On day 40 of gestation, conversion of the ectomeninx mesenchyme into cartilage constitutes the beginning of the formation of the chondrocranium. Fusion of 2 presphenoid cartilages forming the precursor to the presphenoid bone occurs by week 8 of gestation. These will form the anterior part of the sphenoid bone. The chondrification centers of the orbitosphenoid (lesser wing) and alisphenoid (greater wing) will also contribute to the wings of the sphenoid bone. Endochondral and intramembranous ossification centers for the greater wings of the sphenoid appear around 8 weeks' gestation. Intramembranous ossification of the squamous portion of the temporal bone also begins in week 8 of gestation. Clearly, by week 8, a preliminary design for the cartilaginous cranial base has already been established; therefore, if a vascular lesion is to primarily disrupt this architectural design, it must occur after week 2, when molecular mechanisms for the process of angiogenesis are established, and before or during weeks 4–8, the critical timeframe in craniofacial development.
Of interest, there is a resemblance between the CT images in our patients and those with osseous-dural defects of the skull base that have been associated with spontaneous CSF leaks, notably in obese middle-aged women.24 The pathophysiology of this phenomenon is thought to be due to chronically increased intracranial pressure subsequently causing arachnoid granulations to increase in size and embed themselves in pockets within the calvaria. With time, these arachnoid granulations thin the dura mater, and rupture of the dura may eventually lead to a CSF leak. None of our patients demonstrated an active CSF leak, and we are not aware of any association of CSF leaks with venous malformations of the head and neck. Furthermore, none of our patients were obese or had papilledema or an empty sella. Given the similarities in bony findings in our patients and in those with spontaneous CSF leaks, however, we should consider the possibility that whatever embryologic error leads to venous malformation may also lead to dural deficiency and an osseous-dural defect resulting in a CSF leak—a potential complication for which we will continue to observe our patients.
In our cases, we hypothesize that the presence of the venous malformation is the primary developmental error, disrupting the development of the chondral cartilage of the skull base and resulting in a focal osseous defect in the cranial base. Given that venous malformations of the head and neck are uncommon but not rare, it is unclear why we do not more frequently observe focal bone defects of the squamosa of the temporal bone and greater wing of the sphenoid bone in association with venous malformations of the head and neck. We have identified 3 cases with this association during 5 years in our own practice; however, we do not think that it is a chance association. We hope that increased recognition of this association will lead to identification of additional cases for future study.
References
- Received January 24, 2010.
- Accepted after revision January 27, 2010.
- Copyright © American Society of Neuroradiology