Abstract
SUMMARY: Advances in brain imaging technologies allow a more comprehensive means to investigate pediatric neurological disorders and diseases. This article highlights 5 unique projects in pediatric neuroradiology which may improve our understanding of these disorders.
Abbreviations
- ASP
- autism spectrum disorders
- CTDIvol
- CT dose index volume
- DLP
- dose-length product
- ICA
- independent component analysis
- MEG
- magnetoencephalography
- mTBI
- mild traumatic brain injury
- RS-fMRI
- rest-state functional MR imaging
- SCD
- size-corrected dose
- TBI
- traumatic brain injury
- TSC
- tuberous sclerosis complex
During the past decade, technologic advances in neuroimaging have been taking place at a phenomenal rate for a wide range of pediatric diseases and disorders of the central nervous system. Nowhere is this more evident than in the impressive research being undertaken by the investigators featured in this article. Although there are literally hundreds of innovative neuroimaging studies underway in this country and around the world, I have chosen to highlight 5 well-established research projects that, separately and in combination, promise to impact the field of pediatric neuroimaging in very significant ways both in the short and long term. It is impossible in the limited amount of space here to do justice to these 5 projects, but we hope you will find each summary sufficiently intriguing to learn more by reading the recent peer-reviewed articles published by these accomplished investigators, which are included in this article for your reference.
Neuroimaging Advances in Understanding Autism
Timothy A. Roberts, PhD, Department of Radiology, Children's Hospital of Philadelphia, Philadelphia, Pennsylvania; e-mail: ROBERTSTIM{at}email.chop.edu. Funding: 5NIH R01-DC008871.
Recently, Dr Tim Roberts has been leading investigations into abnormal auditory processing in children with ASD. Funded by 5NIH R01-DC008871, as well as several charitable foundations, this work has centered on the definition of electrophysiologic signatures or biomarkers that might be used diagnostically and prognostically or as a conduit to experimental models and ultimately as a measure of response to therapy. Three key articles have recently emerged—1 focused on the detection by MEG (Fig 1A) of a split-second delay in children with ASD, in the 100-ms response of the auditory cortex (Fig 1B) to simple auditory stimulation (Fig 1C).1 This measure and associated reduction in γ-band oscillatory phase synchrony has also been used by Roberts to lend credence to (and lead toward validation of) a valproate-induced mouse model of autism, a model in which therapeutic interventions can improve both these biomarkers and also behavior,2 paving the way for future clinical pharmaceutical trials in ASD. Recently, extension of these early auditory cortex responses to later MEG-detected mismatch field responses (which are neural signatures of “change detection”) have shown delays in children with autism of up to 50 ms, with the greatest delays in the group with clinically significant language impairment,3 suggesting that heterogeneity across the autism spectrum may be resolvable by neural signatures. Ongoing work seeks to associate “timing” parameters of neurophysiology in ASD with underlying white matter structural development.4
A, Child undergoing magnetoencephalography. B and C, Split-second delay in children with ASD, in the 100-ms response of the auditory cortex.
Alliance for Radiation Safety in Pediatric Imaging (Image Gently Program): Size-Corrected Dose (A New Patient Dose Estimate) and First Pediatric National Registry for CT Scans
Marilyn Goske, MD, Department of Radiology, Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio; e-mail: marilyn.goske{at}cchmc.org
American Association of Physicists in Medicine Task Group 204: Size-Corrected Dose, A New Patient Dose Estimate
One of the unique challenges for pediatric radiologists has been the lack of a meaningful estimate of patient dose based on the patient's size. While the average dose delivered to the scan volume for a specific examination, which is a derivative of CTDI vol and DLP, is provided to the radiologist in the CT dose report at the end of the scan and is often displayed in the PACS, these metrics reflect the radiation production of the CT scanner to a standard phantom (either 16 or 32 cm in diameter) and do not reflect the patient dose. A significant sign of progress has been the formation of Task Group 204 by the American Association of Physicists in Medicine, chaired by medical imaging physicists John Boone, PhD, and Keith Strauss, MSc. At the request of pediatric radiologists in the past5 and, specifically, the Alliance for Radiation Safety in Pediatric Imaging (the Image Gently campaign) (Fig 2A),6 the Task Group has developed a scientifically validated correction factor that can be applied to the CTDIvol displayed by the CT scanner to calculate the SCD, a better estimate of pediatric patient dose.7 This practical tool will help radiologists estimate radiation dose for children undergoing medical imaging. The conversion factor developed by this task group is provided in a series of simple look-up tables that can, within 10%–20%, estimate the patient dose received at the time of a CT scan. All that is required is the knowledge of CTDIvol, patient size, and size of the CTDI phantom assumed by the CT scanner to calculate the CTDIvol. The calculated SCD is a more meaningful dose estimate to place in the radiology report for pediatric patients than CTDIvol or DLP. The report will provide an example of how to use this correction factor and suggested language to use in the report by radiologists.
A, Logo for the Alliance for Radiation Safety in Pediatric Imaging (the Image Gently campaign). B, Logo for the Quality Improvement Registry for CT Scans in Children.
First Pediatric National Registry: Quality Improvement Registry in CT Scans in Children
Despite the fact that there are between 7 and 11 million CT scans obtained in children annually, there is little information regarding what radiation dose pediatric patients are receiving nationally.8 In addition, as opposed to the European Union, the United States medical imaging community has lagged behind in its effort to develop diagnostic reference levels, an important and necessary next step in dose optimization in CT scans for children.9 Through the support of an Education Scholar grant from the Radiological Society of North America for the principal investigator and the support of the National Radiology Data Registry of the American College of Radiology, a consortium of 6 children's hospitals (Cincinnati Children's Hospital Medical Center, Children's Hospital Boston, Children's Hospital of Philadelphia, Duke Medical Center, Massachusetts General Hospital, and Primary Children's Hospital) have formed a prototype registry. The registry is initially focusing on CT scans of the abdomen (Fig 2B). One of the key goals of the registry is to develop quality-improvement teams at each site to standardize CT technique across the 6 sites and use quality-improvement methodology to implement change. It is expected that after the initial test phases of this registry, it will be expanded to include other sites and CT examination types.
Rest-State fMRI in Pediatric Patients with Intractable Epilepsy
Byron Bernal, MD, Nolan Altman, MD, Department of Radiology, Miami Children's Hospital, Miami, Florida; e-mail: byron.bernal{at}mch.com.
RS-fMRI is an important recent development in fMRI with unique features that make the procedure quite suitable for the pediatric population. RS-fMRI does not require the use of any task to demonstrate brain activation. RS-fMRI is a generic term that describes methods to mine data of basal brain function. Some of these methods are ICA, seed-based brain connectivity, and regional homogeneity. ICA seems to be more suitable for clinical purposes due to the ease in being obtained.
Several cortical components that are usually bilaterally represented (canonical components) have been described in healthy adult10 and young subjects,11 including primary and secondary visual, motor, and auditory areas; and angular gyrus, precuneus, cingulate gyrus, prefrontal areas, and dorsoparietofrontal areas.
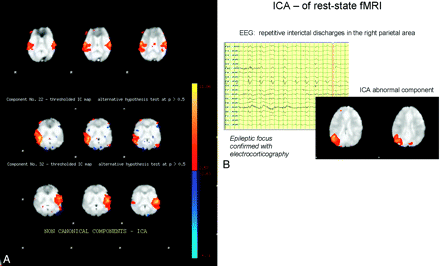
These investigators are currently looking at cortical components in patients with intractable epilepsy. In these patients, the auditory canonical activation may be bilateral as is seen in the healthy population (Fig 3A, upper row). Hemispheric dissociation of canonical components, however, may also be seen in >50% of patients with intractable epilepsy (Fig 3A, middle and lower row). Focal regions of activation that are not expected (noncanonical components) have also been found. Investigators have found that in some cases of epilepsy, these abnormal components correspond to the epileptogenic region (Fig 3B).
A, Dissociation of canonical components. Upper row shows a typical canonical temporal (auditory) independent component, characterized by bilateral “activation.” Middle and lower rows show 2 separate dissociated components of activation seen in a patient with intractable epilepsy. B, Abnormal right parietal component in a patient with an epileptic focus demonstrated in the same area.
RS-fMRI is in rapid development, and this technique may become a potential biomarker of global brain function with its usage in epilepsy a work in progress.
Changing the “Rules of Engagement” in Pediatric Mild Traumatic Brain Injury
Jill V. Hunter, MD, Department of Radiology, Texas Children's Hospital, Houston, Texas; e-mail: jvhunter{at}texaschildrens.org. Funding: PO1NS056202 NINDS/NIH.
TBI is the leading cause of death and acquired disability in childhood.12 Adolescents are frequently affected by mTBI, often related to sports injuries. The incidence of TBI-related emergency department visits has been reported at 757 per 100,000 in the 15- to 19-year age range.13 This is likely a significant underestimate of the true incidence because an unknown number of cases never present for medical attention. There is a growing awareness of the cognitive, emotional, and even physical decline that can accompany repetitive brain trauma as demonstrated by the recent passage of the Zackery Lystedt law, H.R. 1824 in 2009, prohibiting youth athletes suspected of sustaining a concussion from returning to play without a complete evaluation and written consent from a licensed healthcare professional trained in the evaluation and management of concussions.13
Neuroimaging has the potential to identify youth at the greatest risk. While CT is currently the mainstay of screening for suspected brain trauma, MR imaging has been increasingly used by investigators to better understand the underlying pathophysiology behind mTBI. Preliminary studies that require ratification have suggested, with the use of 30-direction diffusion tensor imaging, that restricted diffusion abnormalities may be detected acutely following mTBI.14 This is in the absence of hemorrhage as confirmed by susceptibility-weighted imaging, which, of itself, has been shown to be a predictor of poor pediatric outcomes.15 Mitochondrial disruption resulting in cytotoxic edema from sodium pump failure is 1 postulated mechanism for this observation. Longitudinal studies are now underway to correlate cognitive testing with the findings of this and other acute MR imaging parameters. If the results of these studies prove predictive of outcomes, then consideration might be given to MR imaging screening for mTBI in the future, thereby avoiding concerns regarding radiation dosage.
Diffusion Imaging Provides Insight into White Matter Microstructural Integrity in Tuberous Sclerosis Complex
Simon K. Warfield, PhD, and Benoit Scherrer, PhD, Computational Radiology Laboratory, Department of Radiology, and Jurriaan Peters, MD, and Mustafa Sahin, MD, PhD, Department of Neurology, Children's Hospital Boston, Boston, Massachusetts. e-mail: simon.warfield{at}childrens.harvard.edu. Funding: This research was supported in part by the Harvard Clinical and Translational Science Center and by NIH R01 RR021885, R01NS058956 and P30HD18655.
The disruption of brain connectivity may be an important feature underlying abnormal brain function in a number of neurologic disorders. Diffusion imaging can be used to probe white matter microstructure and connectivity in the brain.16 It is based on estimating the underlying fiber-orientation distribution function, which characterizes the orientation of white matter fiber bundles passing through each voxel. A popular model of the fiber-orientation distribution function has been the diffusion tensor model, which describes the magnitude and direction of water molecule diffusion with a tensor at each voxel. It enables the assessment of fiber bundle characteristics in addition to the brain connectivity. Certain diffusion tensor measures correlate with myelination, axonal integrity, attenuation, and organization or coherence.16,17
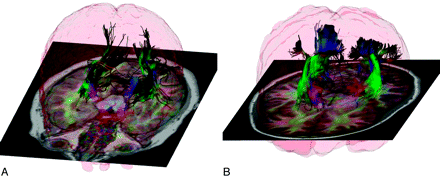
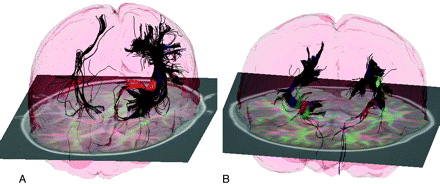
TSC is a neurocutaneous autosomal dominant genetic disorder. Patients with TSC are often also diagnosed with autism, epilepsy, and developmental delay. Because TSC is frequently detected early in life, it provides a unique opportunity to study autism, epilepsy, and developmental delay. The TSC mutations have been associated with growth of excessive connectivity18 and with the loss of navigation cues for the developing axons.19 We have developed strategies for imaging pediatric subjects that allow us to characterize multiple fiber bundles per voxel with short-duration MR imaging.20,21 In applying diffusion imaging to healthy controls and patients with TSC, we developed sophisticated tractography methods to assess connectivity and to allow us to assess the microstructural integrity of the white matter in the connected regions.22 The patients with TSC showed disorganized structurally abnormal white matter fiber tracts, particularly in the visual and social cognition areas of the brain (Figs 4 and 5). Increases in radial diffusivity suggest that these tracts were also missing some of the fatty myelin coating that helps conduct electrical signals.23
Brain miswiring in tuberous sclerosis and autism. A and B, These diffusion tensor tractography images of the brain, analyzed and visualized with the CRKit software developed in the Computational Radiology Laboratory at Children's Hospital Boston, Massachusetts, illustrate the disorganization of white matter fibers in a 17-year-old girl with TSC and autism (A) compared with a healthy girl of the same age (B). In the affected girl, the white matter fiber bundles that carry information from the thalamus of the brain to the visual cortex are less organized, with far fewer axons connecting to the cortex. The brighter colors in the healthy brain illustrate greater structural integrity of the tracts.
Brain miswiring, tuberous sclerosis, and developmental delay. A and B, These diffusion tensor tractography images of the brain illustrate the disorganization of white matter in a 5-year-old girl with TSC and developmental delay (A) compared with a healthy girl of the same age (B). In the affected girl, the white matter fiber bundles that carry information from the thalamus of the brain to the visual cortex are strikingly asymmetric, with far fewer axonal connections on the right side of the brain (which appears at left in these images).
References
- Copyright © American Society of Neuroradiology