Abstract
BACKGROUND AND PURPOSE: Cerebral vasospasm resistant to medical management frequently requires intra-arterial spasmolysis. Angiographic resolution of vasospasm does not provide physiologic data on the adequacy of reperfusion. We recorded pre- and postspasmolysis PbO2 data in the endovascular suite to determine whether this physiologic parameter could be used to determine when successful reperfusion was established.
MATERIALS AND METHODS: Eight patients with 10 Licox monitors and cerebral vasospasm underwent intra-arterial spasmolysis. Pre- and postspasmolytic PbO2 was recorded for comparison. Other physiologic parameters, such as CPP, ICP, SaO2, and Fio2, were also recorded.
RESULTS: Mean prespasmolysis PbO2 recordings were 35.2 and 27.3 for the mild-to-moderate and moderate-to-severe vasospasm group, respectively. Mean postspasmolysis PbO2 increased to 40.3 and 38.4, respectively, which was statistically significant (P < .05) for both groups. In 100% of instances in the moderate-to-severe group and 83% of instances in mild-to-moderate group, the mean PbO2 increased after spasmolysis and correlated with improvement in angiographic vasospasm. Other physiologic parameters, such as CPP, ICP, SaO2, and Fio2, did not show any statistically significant difference before and after spasmolysis.
CONCLUSIONS: PbO2 monitoring provides the interventionalist with an objective physiologic parameter to determine adequate spasmolysis. Further investigation is needed to establish target PbO2 rates indicative of adequate reperfusion, which can be used in the endovascular suite.
ABBREVIATIONS:
- ACA
- anterior cerebral artery
- CPP
- cerebral perfusion pressure
- CV
- cerebral vasospasm
- Fio2
- fraction of inspired oxygen
- ICP
- intracranial pressure
- ICU
- intensive care unit
- PbO2
- partial brain tissue oxygen tension
- SaO2
- arterial oxygen saturation
- SE
- standard error
CV frequently results in devastating neurologic outcomes limiting functional recovery and a return to an independent lifestyle after aneurysmal SAH. More than 30% of patients will develop severe symptomatic CV and delayed neurologic deficits after SAH, but this number has been reported by Fisher et al1 to exceed 90% in those with large clot burdens. In severe cases of CV, traditional medical management protocols by using “triple-H” therapy (hypertension, hypervolemia, hemodilution) will fail to establish adequate reperfusion and will require emergent intra-arterial spasmolytic therapy in the endovascular suite.2,3
Currently, the efficacy of spasmolytic therapy and the adequacy of reperfusion are established by a subjective comparison of pre- and posttreatment angiography rather than by objective physiologic parameters. This method is of concern when large-vessel spasm has resolved and the interventionalist notices sluggish flow in the distal arterial tree suggestive of small-vessel spasm, making it difficult to assess whether adequate perfusion has been truly re-established.
On this premise, we hypothesized that measured PbO2 would correlate with the severity of large-vessel arterial vasospasm, would increase after spasmolytic therapy, and would correlate with angiographically confirmed improvement in CV.
Materials and Methods
Approval was obtained for this study from our institutional review board at SUNY Upstate Medical University. All charts and radiographic images were reviewed retrospectively for this study.
General Patient Population
Eight patients with poor-grade aneurysmal SAH (Hunt and Hess scale 4–5 or grade 3 who later deteriorated clinically from CV), who were admitted to our neuroscience ICU and required ICP monitoring and external ventricular drainage, also underwent Licox intraparenchymal monitor (Integra Life Sciences, Plainsboro, New Jersey) placement when CV was detected radiographically. Licox monitors were placed unilaterally for radiographic findings of focal CV (6 patients) and bilaterally for diffuse CV (2 patients). Eight patients (with a total of 10 Licox monitors) in this study underwent 29 cerebral angiograms for the diagnosis and treatment of CV.
Licox Insertion and Management
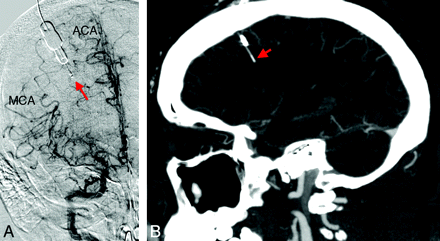
Because of their neurologic conditions, all patients with SAH in this review required mechanical ventilation and sedation so that complete and reliable neurologic assessments could be performed consistently. The Licox monitor was placed to continuously monitor ICP, brain temperature, and PbO2. All Licox monitors were placed at the Kocher point according to standard protocol for intraparenchymal catheter placement positioned at the ACA-MCA watershed territories (Fig 1A), about 2 cm deep into the brain parenchyma (Fig 1B). The Licox monitor was placed on the side where the cerebral angiogram demonstrated CV.
A, Digital subtraction angiography demonstrating the tip of the Licox monitor in the ACA-MCA watershed territory. B, Reconstructed sagittal CT angiogram demonstrates the intraparenchymal location and depth of the fiberoptic Licox cable tip.
Licox readings were recorded hourly during routine data collection. The mean of six 1-hour readings before and after angiography was used for data analysis, totaling a 12-hour window. We included 6 readings at 1-hour intervals to minimize the chances of overlapping CV episodes because some patients underwent intra-arterial spasmolytic therapy twice daily. The Licox readings were monitored continuously during the intervention as well; however, to prevent changes in head elevation affecting our data readings, the Licox data analyzed in this study included only those readings obtained when the patient's head was at 30°–45°, to avoid changes in PbO2 that could result from changes in CPP with differences in head positioning. None of the readings during patient transport and positioning formed part of this data analysis.
CPP, ICP, SaO2, and FiO2 were also recorded hourly and 6 readings at 1-hour intervals before and after the spasmolysis therapy were analyzed along with PbO2. Partial pressure of arterial oxygen (PaO2) was not routinely checked before and after cerebral angiography as per our standard of care. The patients had daily routine labs including complete blood count; if their hematocrit level dropped to <30, they were transfused to maintain a hematocrit level ≥30.
Routine Screening for CV
Daily transcranial Doppler studies and CT angiography and CT perfusion studies on postbleeding days 4, 6, and 10 were routinely performed to screen for CV. If CV was suspected on the basis of these imaging modalities, the patient was brought to angiography for confirmation; if already failing maximal medical treatment, the patient underwent intra-arterial spasmolysis.
Management of CV
Our medical management protocols are as follows: In response to falling PbO2, the inspired O2 was gradually increased to 100% to improve the PbO2. If this was unsuccessful or only transiently successful, then cerebral angiography was performed after propofol or midazolam (Versed) infusions, the standard sedating agents used in our ICU. The patients included in this study were already on these medications while they were kept sedated and ventilated in the ICU. Once CV was confirmed on angiography, spasmolysis was performed by using either intra-arterial pharmaceutical agents (verapamil and nitroglycerin) or a combination of pharmaceutical and mechanical (balloon angioplasty) techniques, based on the preference of the interventionalist.
Angiographic Parameters for Measuring CV
Angiographic parameters used to determine the severity of CV were based on the percentage of stenosis of the parent artery: mild-to-moderate spasm (<50% arterial stenosis) and moderate-to-severe spasm (≥50% arterial stenosis). PbO2 levels before and after spasmolytic therapy were determined in both groups.
Data Analysis
Pre- and postspasmolysis PbO2 and physiologic parameters, including CPP, ICP, SaO2, and Fio2, were analyzed by using the paired-samples t test; statistical significance was defined as P < .05. Data analysis was performed by using the commercially available Statistical Package for the Social Sciences software, Version 14 (SPSS, Chicago, Illinois).
Results
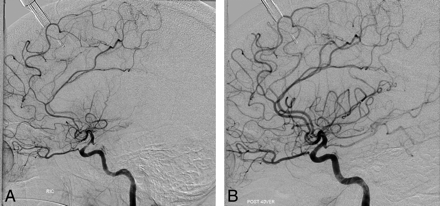
Data from 8 patients with 10 Licox monitors were analyzed in this retrospective study. In these 8 patients, a total of 29 cerebral angiograms were obtained when CV was detected with noninvasive radiographic imaging. In every instance, CV was treated pharmacologically with intra-arterial verapamil or nitroglycerin or with combined pharmaceutical and mechanical spasmolysis (performed in 1 patient with severe supraclinoid ICA CV unresponsive to pharmaceutical spasmolysis). Postspasmolytic angiography demonstrated alleviation of CV in every case (Fig 2A, -B). On the basis of the severity of angiographic CV before spasmolysis, treatment events were categorized into 2 groups: 1) mild-to-moderate CV (<50% arterial stenosis), and 2) moderate-to-severe CV (≥50% arterial stenosis). The percentage improvement in PbO2 in the mild-to-moderate and moderate-to-severe groups was 14% and 40%, respectively (Table).
Prespasmolytic (A) and postspasmolytic (B) cerebral angiograms demonstrating improvement in cerebral vasospasm in ICA, ACA, and MCA vessels after intra-arterial verapamil infusion.
Mild-to-moderate and moderate-to-severe group physiologic parameters before and after spasmolytic therapy along with percentage improvement in PbO2 after spasmolytic therapy
Statistical analysis by using the paired-samples t test demonstrated a statistically significant improvement in PbO2 after intra-arterial spasmolysis for both the mild-to-moderate (P = .002) and the moderate-to-severe groups (P = .000). Other physiologic parameters, such as CPP, ICP, SaO2, and Fio2, and head elevation did not show any statistically significant difference between the prespasmolysis and postspasmolysis periods. We also compared the mean PbO2 between mild-to-moderate and moderate-to-severe groups in the prespasmolysis period, and though the difference was not statistically significant, it did suggest a trend (P = .067).
Mild-to-Moderate Vasospasm Group
Fifteen cerebral angiograms demonstrated mild-to-moderate CV; during 3 of these, patients had bilateral Licox monitors yielding 18 data points for statistical analysis. The Licox monitor showed improved PbO2 after spasmolysis in 15 instances (83%) in this group. During 3 instances, the mean postspasmolytic PbO2 was slightly lower than the prespasmolytic mean PbO2, but this was not statistically significant. During 1 cerebral angiography showing mild-to-moderate CV, Licox monitor malfunction was noticed due to unstable readings and was excluded from the data analysis.
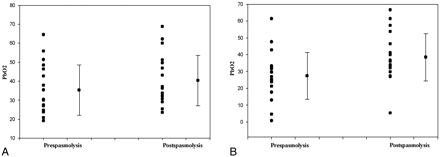
Mean PbO2 in the prespasmolytic and postspasmolytic period was 35.2 ± 3.1 mm Hg (mean ± SE) and 40.3 ± 3.1 mm Hg (mean ± SE), respectively (Table and Fig 3A). The paired difference between groups by using the 2-tailed paired-samples t test was 5.0 ± 1.4 (mean ± SE). This improvement in PbO2 after spasmolysis was statistically significant (P = .002) (Table and Fig 3A). Mean CPP, ICP, SaO2, and Fio2 did not have any statistically significant differences in prespasmolytic and postspasmolytic periods (Table).
Point plot and column means graph displaying the PbO2 before and after spasmolysis in the mild-to-moderate vasospasm group (A) and moderate-to-severe vasospasm group (B). PbO2 values during the different cerebral angiographies are plotted as points along the y-axis with the mean and SD next to the point plot for both groups. Statistical significance (P < .05) was attained between pre- and posttreatment in both groups.
Moderate-to-Severe Vasospasm Group
Fourteen cerebral angiograms demonstrated moderate-to-severe CV. During 5 of these angiographies, 2 patients had bilateral Licox monitors yielding 19 data points for statistical analysis. The Licox monitor showed improved PbO2 after spasmolysis during all 19 instances (100%) in this group (Fig 3B). In this group also, the Licox monitor malfunction was noticed during 1 cerebral angiography and hence was excluded from the data analysis.
The mean PbO2 in the prespasmolytic and postspasmolytic period was 27.3 ± 3.1 mm Hg (mean ± SE) and 38.4 ± 3.2 mm Hg (mean ± SE), respectively (Table and Fig 3B). The paired difference between groups by using the 2-tailed paired-samples t test was 11.0 ± 2.0 (mean ± SE). This improvement in PbO2 after spasmolysis was statistically significant (P = .000) (Table and Fig 3B). The mean CPP, ICP, SaO2, and Fio2 did not show any statistically significant differences in the pre- and postspasmolysis period (Table).
Spasmolytic Therapy
Of the 31 angiographies, balloon angioplasty had to be used only once for pharmacologically resistant vasospasm in the supraclinioid ICA in a patient with severe CV. Verapamil alone was used to treat vasospasm during 25 angiographies and was supplemented with nitroglycerine during 3. Nitroglycerine was used as the only vasodilating agent in 3 patients. The improvement in PbO2 after spasmolytic therapy was found to be sustained from 8 hours in 1 patient with severe CV requiring twice daily spasmolysis to an indefinite period in another whose CV had improved after 1 treatment and did not necessitate repeat treatment.
Discussion
Currently, there are no physiologic parameters in the interventionalist's armamentarium to define adequate reperfusion after spasmolytic therapy in patients with SAH. The Licox intraparenchymal brain tissue oxygen monitor is a novel adjunct to spasmolytic therapy, allowing continuous real-time assessment of reperfusion during spasmolysis in the interventional suite. In this study, improvement in PbO2 recorded with the Licox monitor strongly correlated with the resolution of CV on angiography. Until now, determination of appropriate spasmolytic therapy in the angiography suite was usually subjective based on the angiographer's interpretation of the images, without any objective physiologic parameters to define an end point for adequate reperfusion. Even though arterial diameter can be used to objectify the severity of CV, there is no standardized measurement of arterial stenosis to define mild, moderate, or severe CV. We categorized CV into mild-to-moderate (<50% large-vessel arterial narrowing) and moderate-to-severe (≥50% large-vessel arterial narrowing). Additionally, there is no method to determine whether adequate restoration of brain oxygenation has been established on the basis of imaging studies alone, particularly in the setting of distal CV. The interventionalist is frequently left to interpret blood flow by timing the transition time from the arterial to venous phases.
Monitoring brain oxygenation provides the interventionalist with a novel technique to objectify the adequacy of reperfusion and to determine whether the CV was successfully treated or whether further intervention is needed. Some have reported that both spasmolysis with papaverine and oral nimodipine doses reduced PbO2.4⇓–6 These findings, however, are likely secondary to reduced CPP in response to these vasodilating agents. In our study however, the mean PbO2 improved after spasmolysis in both mild-to-moderate and moderate-to-severe groups. Although the improvement in PbO2 was found to be statistically significant in both groups, the effect was more pronounced in the moderate-to-severe group. This improvement in PbO2 correlated with the resolution of angiographic vasospasm. In the moderate-to-severe group, PbO2 readings in the postspasmolytic period showed improvement during all instances. In the mild-to-moderate group, there were 3 instances in which postspasmolytic PbO2 readings were slightly lower than the prespasmolysis readings. In these 3 instances, the difference in pre- and postspasmolytic PbO2 was small (mean difference ≅ 2 mm Hg) and not statistically significant (P > .05). The PbO2 was already >25 mm Hg in the prespasmolytic period in all 3 instances, which is within the normal range based on our knowledge from the literature on brain tissue oxygen monitoring in patients with traumatic brain injury.7 Thus, we believe that their PbO2 did not change significantly after the interventional cerebral angiogram.
During 2 cerebral angiographies, 1 each in the mild-to-moderate and moderate-to-severe groups, the Licox monitor malfunction was noticed due to unstable readings; these monitors were replaced. The data from these 2 cerebral angiograms were excluded from the final data analysis. Our data also showed a strong trend in the PbO2 and the severity of CV on angiography. PbO2 was lower in the moderate-to-severe group than in the mild-to-moderate group in the prespasmolytic period (P = .06).
Limitations of using an invasive PbO2 probe such as the Licox include a small volume of tissue (within a 7.1 mm2 area of the catheter tip) that can be monitored with a single probe.8 This places severe limitations on the volume of brain that can be monitored for CV, which can be a diffuse cerebral entity. Also, positioning of the Licox tip remains controversial with regard to the depth and location of placement. We routinely place the Licox through a burr-hole at the Kocher point (11 cm posterior to the glabella, 2–3 cm lateral to midline, in the midpupillary line) and insert the cable tip to approximately 2 cm from the surface and confirm positioning with a CT of the head. The Kocher point serendipitously is located near the anterior ACA-MCA watershed territory and should allow detection of CV in both the distal ACA and MCA territories (Fig 1A). Patients with severe SAH or demonstrable bilateral CV can receive bilateral Licox monitors so that both cerebral hemispheres can be monitored for CV.
Developing techniques to monitor PbO2 over larger volumes of brain tissue is needed to improve the utility of this technique in the endovascular suite. Thermal diffusion microprobes have been implanted to record regional cerebral blood flow changes, but these too have the disadvantage of being invasive and indirectly measure flow and oxygenation, making interpretation difficult and dependent on external factors that are difficult to control.6 Even more beneficial would be technologic advancements in noninvasive brain tissue oxygen monitoring that would allow simultaneous recordings over the entire cortical surface. The advantage of the Licox over currently available noninvasive brain oxygen monitoring modalities, such as transdermal infrared technology, is its accuracy and fewer external variables that would potentially interfere with O2 detection.9⇓⇓⇓⇓⇓⇓⇓–17 Contemporary noninvasive monitoring systems have failed to accurately monitor brain O2 levels because of external variables such as ambient light and poor skin-to-electrode contact, both of which interfere with accurate monitoring.18,19
This study does have inherent limitations including its retrospective nature and small sample size. A multicenter prospectively randomized controlled trial with a larger number of patients would improve our ability to determine the utility of brain tissue oxygen monitoring as an objective physiologic parameter for determining adequate cerebral reperfusion after intra-arterial spasmolytic therapy in the endovascular suite.
Conclusions
We have demonstrated a trend between PbO2 levels and the severity of CV in patients with SAH. Our data suggest that the PbO2 level for mild-to-moderate CV is approximately 35 ± 3 mm Hg, and for moderate-to-severe CV, 27 ± 3 mm Hg. Resolution of CV on angiography strongly correlated with increased PbO2 levels, and the differences between pre- and posttreatment PbO2 were statistically significant. PbO2 is a useful physiologic parameter for determining CV in the ICU and a useful tool for determining adequate restoration of cerebral blood flow after spasmolytic therapy.
Acknowledgments
We acknowledge the work of the nursing staff in the Neurosciences Intensive Care Unit at SUNY Upstate Medical University Hospital who cared for these critically ill patients and were responsible for the transportation of the equipment used during this study. We also acknowledge the work of Mariam Donohue, PhD, student at SUNY Upstate Medical University, for critically appraising the manuscript.
Footnotes
-
Disclosures: Eric Deshaies—UNRELATED: Consultancy: ev3, Integra, Comments: physician consultant for both companies. No consultant services related to this manuscript. I was not a consultant during preparation of the initial manuscript when data were reviewed or when the manuscript was initially submitted.
References
- Received September 6, 2011.
- Accepted after revision October 25, 2011.
- © 2012 by American Journal of Neuroradiology