Abstract
BACKGROUND AND PURPOSE: Radiation exposure from neurointerventional procedures can be substantial, with risk of radiation injuries. We present the results of a follow-up program applied to potential skin injuries in interventional neuroradiology based on North American and European guidelines.
MATERIALS AND METHODS: The following guidelines approved in 2009 by SIR and CIRSE have been used over the last 2 years to identify patients with potential skin injuries requiring clinical follow-up: peak skin dose >3 Gy, air kerma at the patient entrance reference point >5 Gy, kerma area product >500 Gy · cm2, or fluoroscopy time >60 minutes.
RESULTS: A total of 708 procedures (325 in 2009 and 383 in 2010) were included in the study. After analyzing each dose report, 19 patients (5.9%) were included in a follow-up program for potential skin injuries in 2009, while in 2010, after introducing several optimizing actions and refining the selection criteria, only 4 patients (1.0%) needed follow-up. Over the last 2 years, only 3 patients required referral to a dermatology service.
CONCLUSIONS: The application of the guidelines to patient radiation dose management helped standardize the selection criteria for including patients in the clinical follow-up program of potential skin radiation injuries. The peak skin dose resulted in the most relevant parameter. The refinement of selection criteria and the introduction of a low-dose protocol in the x-ray system, combined with a training program focused on radiation protection, reduced the number of patients requiring clinical follow-up.
ABBREVIATIONS:
- CIRSE
- Cardiovascular and Interventional Radiology Society of Europe
- DAP
- dose-area product
- DOLIR
- dose on-line for interventional radiology
- ICRP
- International Commission on Radiologic Protection
- SIR
- Society of Interventional Radiology
In recent years, the benefits and complexity of interventional procedures have increased substantially and the radiation dose to patients and staff is a matter of concern.1⇓–3 Among medical specialties, interventional neuroradiology is of utmost benefit to patients, but it uses high radiation doses in a significant number of procedures. Radiation injuries in patients may occur and should be listed as a risk in the informed consent.2,4 In their quality programs, interventional radiology units include the evaluation of patient radiation doses and the criteria for including patients in a clinical follow-up as part of postprocedural care when relevant dosimetric parameters indicate potential skin radiation injuries.2,4
In 2000, the ICRP published a set of recommendations on how to avoid radiation injuries in medical interventional procedures.2 These recommendations have been developed in several guidelines and adopted by interventional radiology medical societies.4
The European Directive on Medical Exposures 97/43/Euratom5 requires Member States of the European Union (article 9) to use appropriate radiologic equipment submitted to quality assurance programs and to assess patient doses. Preventing high-dose exposures with diagnostic equipment is another requirement (article 11).
Many patients are still neither being counseled on radiation risks nor followed up when radiation doses from difficult procedures may lead to injury. In some interventional procedures, skin doses to patients exceed the threshold for deterministic effects.6 Several evaluations of cumulative skin dose have been made in interventional neuroradiology procedures.7⇓⇓⇓⇓⇓–13 Radiation-induced skin injuries can sometimes occur after a clinically complex procedure, but may also, on other occasions, result from the use of inappropriate equipment or poor operational techniques. The ICRP states that acute radiation doses may cause erythema at 2 Gy and delayed skin necrosis at 12 Gy.2 Other more recent reports have analyzed the different dose thresholds for radiation injuries in interventional procedures.6
The term “cumulative skin dose,” introduced by the ICRP,2 is used in this article as the value of the dose in air, accumulated at the entrance of the patient during the whole interventional procedure. It is obtained from the air kerma accumulated at a specific point in space relative to the fluoroscopic gantry (called “patient entrance reference point” by the International Electrotechnical Commission)14 during the full procedure, and by taking into account the calibration factor of the ionization transmission chamber of the x-ray system and the attenuation of the table and head support and the increase caused by the backscatter factor.
The accumulated air kerma displayed by the x-ray system and presented in the patient dose reports includes neither the backscatter nor the attenuation of the table, nor the patient's head support. The dose values reported by the x-ray systems also need to be corrected by the corresponding calibration factor of the internal transmission ionization chamber. The “cumulative skin dose” is not the same quantity as the “peak skin dose” (ie, the highest dose at any portion of a patient's skin during a procedure).
The current standard for the x-ray systems used in interventional radiology14 requires supplying information on the kerma area product (also known as DAP; usually measured in Gy · cm2) and cumulative air kerma (or cumulative dose) at the patient entrance reference point (usually displayed in mGy). The cumulative air kerma is calculated by the x-ray system (free in air) at 15 cm from the isocenter in the focus direction (the “reference point”), and its value, once corrected by the calibration factor of the internal transmission ionization chamber, is similar to the cumulative skin dose ±10% in most of the systems (considering the increase caused by the backscatter of the patient and the attenuation in the table and mattress or patient head support).15,16
The ICRP has recommended that maximum cumulative absorbed doses to the skin approaching or exceeding 1 Gy (for procedures that may be repeated) or 3 Gy (for any other procedure) should be recorded in the patient record and that there should be follow-up procedures for such cases.2
In 2009, SIR (in North America) and CIRSE published a common “Guidelines for Patient Radiation Dose Management,” previously adopted by both societies.4 The document introduced the term “significant radiation dose” as a selected threshold value used to trigger additional dose-management actions. In our hospital, the procedure adopted in the quality assurance program is in accordance with the postprocedural care included in these guidelines, especially concerning dose documentation and patient follow-up.
Several articles have been published on radiation skin injuries in interventional procedures,17⇓⇓⇓⇓–22 but very few, if any, give details on the percentage of patients exceeding the trigger values and requiring clinical follow-up after potential radiation injuries.
The aim of this article is to present the results of the application of the SIR-CIRSE guidelines in interventional neuroradiology and report the percentages of patients requiring clinical follow-up due to potential radiation injuries.
Materials and Methods
The San Carlos Hospital Ethical Committee approved this study under the title “Radiological risks in fluoroscopy guided procedures” (code B-09/20). The x-ray system used in this study was a biplane Allura (Philips Medical Systems, Best, the Netherlands). The system was equipped with a flat imaging detector, with a field of view of 48.4 cm (diagonal dimension) for the frontal C-arm and a flat detector of 25 cm (diagonal dimension) for the lateral C-arm.
The frontal C-arm (48 cm) allows for acquiring rotational and CT-mode series in addition to fluoroscopy and DSA acquisitions (with a large variety of protocols for the different clinical procedures and areas examined). The FOVs available for frontal C-arm detectors are 48, 42, 31, 26, 22, 19, and 15 cm. The lateral C-arm detector (25 cm) has 3 FOVs: 25, 20, and 15 cm. The standard configuration of the system has 3 fluoroscopy modes (all at 15 pulses/second) with added filtration of 1 mm aluminum and 0.9, 0.4, and 0.1 mm of copper for low, normal, and high fluoroscopy modes, respectively.
When the procedures are over, a complete patient dose report is produced by the x-ray system and sent by e-mail (intranet) to the medical physics service, where automatic software (DOLIR)23 processes the report and, after including the calibration/correction factors, introduces the relevant dosimetric parameters into a data base and into a graphic interface.
The x-ray system is submitted to a quality assurance program and patient dose records are archived individually according to national regulations. The medical physics service is in charge of the quality control of the x-ray and imaging system, dose calibrations, dose records, and patient and staff radiation protection. This service also gives support to the interventional neuroradiology unit to optimize some imaging protocols.
As part of a full optimization program, and as suggested by the medical physics service and the neuroradiology unit, the service engineers prepared a low-dose protocol for patients, implemented during 2010. It basically consisted of a reduction of patient entrance dose rate in fluoroscopy (decreasing the number of pulses/second with a slight increase of the dose per pulse) and of patient entrance dose per image during the DSA series. Details of this protocol are included in the Results section and compared with the standard protocol.
Part of the optimization program also included a 20-hour training course focused on radiation protection for fluoroscopy-guided procedures, corresponding to the “second level of radiation protection training” as recommended by the World Health Organization,1 ICRP,2,24 and the European Guidelines on Education and Training in Radiation Protection for Medical Exposures.25
In addition, DOLIR was implemented at the hospital during 201023 as an intermediate solution until the DICOM dose-structured reports are available for interventional radiology systems.
Our biplane neuroradiology x-ray system currently has the capability to export, via e-mail, patient dose reports, including fluoroscopy time, DAP, and cumulative dose at the patient entrance reference point for each of the C-arms at the end of each procedure. Patient dose reports also include details on radiographic techniques, geometry values such distances and angulations of the C-arms, and the number of frames of all the DSA series and fluoroscopy runs archived.
Dose values are included by the x-ray system as part of the patient dose reports, but in our software, these have been corrected by the corresponding calibration factor (measured as part of the periodic quality controls of the system) to take into account the accuracy of the internal transmission ionization chamber, by the attenuation of the table and the mattress (relevant for the frontal plane) and by the backscatter factor. The values included in the data base are corrected with all these factors.
Trigger levels for a potential patient follow-up were adapted to the values recommended by the SIR-CIRSE guidelines.4 These values are peak skin dose >3 Gy, cumulative air kerma at the patient entrance reference point >5 Gy, kerma area product >500 Gy · cm2, or fluoroscopy time >60 minutes. The refinement during 2010 consisted of using the cumulative skin dose of each of the planes (frontal and lateral) independently as main trigger levels when one resulted in values >4 Gy, and in taking into account the different angulations of the C-arms during the procedures (included in the patient dose reports) to consider the skin dose distribution.
The medical physics service is alerted when values of DAP or cumulative doses are over these levels. The dose values used are previously corrected by the calibration of the ionization chamber, the attenuation of the table and head support, and the backscatter factor. The information on patient dose is also displayed in the x-ray room during the procedure.
Alerts are analyzed daily by a senior medical physicist, who decides whether a more detailed analysis of the individual dose reports is appropriate. The information is then transferred to the neuroradiology unit, which, in turn, considers whether it is appropriate to give additional information on potential radiation injuries to the patient or to his or her family and to decide on a clinical follow-up. Part of the analysis consists of searching previous procedures existing in the neuroradiology unit data base and considering whether skin doses are relevant. The neuroradiologist in charge of the patient makes further investigations into the patient's clinical records to check whether he or she could have undergone previous procedures in other hospitals. The clinical follow-up procedure has been approved by the hospital's Quality Assurance and Radiation Safety Committee.
Starting a clinical follow-up, as recommended by the SIR-CIRSE guidelines, means extra work that further adds to the workload of informing patients on the reasons for such a clinical follow-up. Therefore, it is only considered after a careful evaluation of the individual patient dose reports by the medical physics service in the case of dose parameters above trigger levels and especially the peak skin dose for the frontal and lateral planes. These peak skin doses are initially assumed (as a conservative hypothesis) to be the cumulative skin dose of each of the planes (frontal and lateral), calculated from the cumulative air kerma included in the patient dose report, corrected by the attenuation of the table and head support, and increased by the backscatter factor (for the frontal plane). The different angulations of the C-arms are also considered to discriminate some cases in which the real skin dose distribution could be very different from the above-mentioned initial conservative hypothesis. The final decision concerning whether to admit patients in a clinical follow-up program lies with the neuroradiology unit.
Results and Discussion
A total of 325 procedures (43% therapeutic, mainly cerebral embolizations) were included in the local data base during 2009, and 383 (40% therapeutic) were included during 2010. From the data base, cases with full patient dose reports were selected for further analysis. It should be noted that, in most interventional suites, patient dose reports are still neither automatically archived nor analyzed. In our center, once the procedure is finished, the operators have to manually send the dose report by e-mail to the medical physics service, where it is processed by the automatic software system DOLIR.22 Some of the dose reports can be lost if the operators fail to send the e-mail. To avoid such mishaps, the manufacturer has been asked to automate e-mails once the procedures are closed.
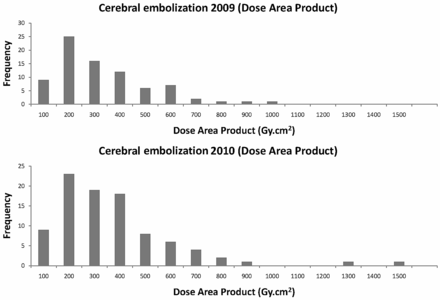
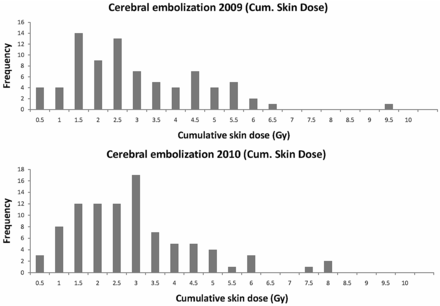
Distributions of DAP and cumulative skin dose values (frontal and lateral planes together) for embolization procedures for the years 2009 and 2010 are presented in Figs 1 and 2. During 2009, the trigger level used for clinical follow-up was the total cumulative dose (>5 Gy in frontal and lateral planes together), but it was considered too conservative for cerebral procedures. Therefore, in 2010, the patient was included in the follow-up program when the cumulative skin dose value was >4 Gy (in one of the planes), and the individual analysis of the C-arm angulations indicated that this conservative hypothesis could be adopted (eg, most of the x-ray beam directions irradiate the same skin area).
Distributions of dose area product values (Gy · cm2) for cerebral embolizations during 2009 and 2010 (frontal and lateral planes together). Median and third quartile values are indicated in Table 1.
Cumulative skin dose distributions (Gy) for cerebral embolizations during 2009 and 2010 (frontal and lateral planes together). Median and third quartile values are indicated in Table 1.
Table 1 presents the results of the patient dose values for cerebral embolizations during 2009 and 2010. The 2009 median values for DAP and cumulative skin dose were very similar to the 2010 ones. There were no statistical differences for either patient dose distribution (P = .643 for DAP, and P = .988 for cumulative skin dose with the Mann-Whitney U test). However, third quartile values were lower in 2010 for cumulative skin dose (3.3 Gy) than in 2009 (3.9 Gy), most likely partly because of the radiation protection training and the application of the low-dose protocol initiated in 2010.
Dose values for cerebral embolizations
The results on skin doses are difficult to compare with other authors because different metrics have been used. We reported cumulative skin doses, obtained from the cumulative air kerma at the patient entrance reference point and displayed by most of the modern x-ray systems,13 while other authors have measured or estimated peak skin doses.7⇓⇓⇓⇓⇓–13 Table 2 (adapted by the authors) compares the results reported by several authors. Our cumulative skin doses and DAPs are in the range of other published values.7⇓⇓⇓⇓⇓–13 In 2009, 19 patients (5.9% of the total number of procedures included in the data base during that year) were included in a follow-up program for potential skin injuries. That year, 20 procedures (2 in the same patient) resulted in cumulative skin doses (frontal and lateral planes together) higher than 5 Gy. In 2010, after introducing optimization actions (ie, a radiation protection training course and a new “low-dose” protocol in the x-ray system), only 11 procedures resulted in a total cumulative dose higher than 5 Gy.
Values of DAP and skin dose for cerebral embolizations
The low-dose protocol (Table 3) was introduced during 2010 as an alternative to the “standard” protocol. Neuroradiologists select the low-dose protocol (especially in the most complex cases) either for part of the procedure or for the full procedure. A numeric evaluation of image quality with test objects was completed before the clinical use of the low-dose protocol, and the neuroradiologists found the differences (in image quality) with the standard mode satisfactory. Dose reduction in fluoroscopy resulted in 30% savings for the low fluoroscopy mode (the most commonly used) and in 47% for DSA images (measured in the conditions indicated in Table 3).
Dose reduction in entrance surface dose rate (with backscatter) measured with a PMMA phantom
After implementing the SIR-CIRSE guidelines, and after considering the peak skin dose values independently for the frontal and lateral planes and taking into account the C-arm angulations during the procedures, only 4 patients (1.0% of the total number of procedures included in the data base during that year) were considered for follow-up. In 2009, 8 patients from the 19 cases (1 of the patients with 2 procedures) included in the follow-up program suffered from temporary alopecia. In 2010, the selection criteria were refined by using the peak skin dose (considered as the cumulative skin dose in each of the frontal and lateral planes and the C-arm angulations), and from the 11 patients with more than 5 Gy (as total cumulative skin dose), only 4 patients were included in the follow-up program (all suffered from temporary alopecia). Only 3 patients (0.4% of the full sample included in the data base) during the 2 years required referral to the dermatology service. In the 2-year period, the maximum patient dose values measured in a single procedure were 1500 Gy · cm2 and 9.5 Gy (cumulative skin dose from frontal and lateral planes together).
Part of the optimization program consisted of the routine application of the following dose reduction rules: using the low fluorocopy mode whenever possible; reducing the fluoroscopy time and the number of DSA images and runs; archiving some of the fluoroscopy runs so as to avoid some DSA series; collimating to the area of interest and using virtual collimation; maintaining the image detector as close as possible to the patient and the x-ray tube as far as possible from the patient's skin (especially relevant for the lateral plane); using magnification only if strictly necessary; and using low-dose protocols, if appropriate.
The median values of these dose distributions are good indexes for drawing comparisons with other similar publications and for moving the project of a future set of “diagnostic reference levels” forward, as recommended by the International Commission on Radiologic Protection26 and the future European Directive on Basic Safety Standards.27
One of the most extensive studies on patient doses for interventional radiology made in the last years is the RAD-IR study in the United States.12 In this study, the group of neuroradiology interventions for the head included embolization of arteriovenous malformations, aneurysms, and tumors. In the US study, a sample of 382 procedures was collected, with a mean patient dose value of 320 Gy · cm2. In our case, mean values for the global 2-year sample (172 procedures identified as “embolizations” in the data base) resulted in 305 Gy · cm2. In any case, median and third quartile values are the best descriptors for highly skewed distributions (Figs 1 and 2).
Limitations of the Study
The clinical complexity of the procedures has not formally been evaluated in this study, but an overview of clinical reports in the neuroradiology unit indicates that the therapeutic procedures were of similar complexity in 2009 and 2010, while the clinical staff of the neuroradiology unit remained unchanged. Despite the calibration factor applied to convert the dose values displayed by the x-ray system to entrance skin dose (including the attenuation of the table and head support and the backscatter factor), inaccuracies can occur in the estimation of the peak skin dose, especially when different C-arm angulations are used during the procedure, when radiation fields overlap, or when tight collimation is used during the procedure causing the real peak skin dose to be lower than the cumulative skin dose in one of the planes.
Conclusions
The application of the SIR-CIRSE guidelines for patient radiation dose management enabled standardization of the selection criteria used to include certain patients in a follow-up program for potential skin radiation injuries. Peak skin dose resulted the most relevant parameter. The selection criteria refinement (using the cumulative skin dose of the frontal and lateral plane independently and the analysis of the C-arm angulations), together with the introduction of a low-dose protocol in the x-ray system and a training program focused on radiation protection, allowed us to reduce the percentage of patients with doses above trigger levels.
Footnotes
Disclosures: Eliseo Vano—RELATED: Payment for Writing or Reviewing the Manuscript: Private language English reviewer.
The authors acknowledge the support of the Spanish grant SAF2009-10485 (Ministry of Science and Innovation).
Previously presented in part as an electronic poster at: CIRSE Congress, September 10–14, 2011; Munich, Germany.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received February 26, 2012.
- Accepted after revision April 29, 2012.
- © 2013 by American Journal of Neuroradiology