Abstract
BACKGROUND AND PURPOSE: Menière disease and idiopathic sudden sensorineural hearing loss can have overlapping clinical presentation and may have similar pathophysiology. Prior studies using postcontrast 3D-FLAIR MR imaging suggest abnormal blood-labyrinth barrier permeability in both conditions, but the 2 diseases have not been directly compared by using the same imaging techniques. We hypothesized that delayed postcontrast 3D-FLAIR MR imaging would show differences in blood-labyrinth barrier permeability between Menière disease and idiopathic sudden sensorineural hearing loss.
MATERIALS AND METHODS: Patients with unilateral Menière disease (n = 32) and unilateral idiopathic sudden sensorineural hearing loss (n = 11) imaged with delayed postcontrast 3D-FLAIR MR imaging were retrospectively studied. Signal intensities of the medulla and perilymph of the cochlear basal turns of both ears in each patient were measured in a blinded fashion. Cochlea/medulla ratios were calculated for each ear as a surrogate for blood-labyrinth barrier permeability. The ears were segregated by clinical diagnosis.
RESULTS: Cochlea/medulla ratio was higher in symptomatic ears of patients with Menière disease (12.6 ± 7.4) than in patients with idiopathic sudden sensorineural hearing loss (5.7 ± 2.0) and asymptomatic ears of patients with Menière disease (8.0 ± 3.1), indicating increased blood-labyrinth barrier permeability in Menière disease ears. The differences in cochlea/medulla ratio between symptomatic and asymptomatic ears were significantly higher in Menière disease than in idiopathic sudden sensorineural hearing loss. Asymptomatic ears in patients with Menière disease showed higher cochlea/medulla ratio than symptomatic and asymptomatic ears in patients with idiopathic sudden sensorineural hearing loss.
CONCLUSIONS: Increased cochlea/medulla ratio indicates increased blood-labyrinth barrier permeability in Menière disease compared with idiopathic sudden sensorineural hearing loss. Increased cochlea/medulla ratio in asymptomatic ears of patients with Menière disease also suggests an underlying systemic cause of Menière disease and may provide a pathophysiologic biomarker.
ABBREVIATIONS:
- BLB
- blood-labyrinth barrier
- CM
- cochlea/medulla
- ISSNHL
- idiopathic sudden sensorineural hearing loss
- MD
- Menière disease
Four-hour delayed intravenous contrast-enhanced inner ear MR imaging is a recently described technique that has been used to image patients with known or suspected Menière disease (MD).1⇓⇓–4 This technique distinguishes the endolymphatic and perilymphatic compartments of the inner ear by allowing dilute contrast to accumulate within the perilymphatic compartment, where the blood-labyrinth barrier (BLB) is permeable, outlining the impermeable endolymphatic compartment. This allows for demonstration of endolymphatic hydrops, the characteristic pathologic alteration in MD. Nevertheless, 10%–33% of patients with MD do not have MR-demonstrable changes of hydrops.2,5,6 This clinical-radiologic discrepancy reflects an incomplete understanding of the disease process and limitations of imaging.
Additional imaging biomarkers of disease activity in MD may be helpful beyond visualization of hydrops. One previous study has explored a relationship between increased BLB permeability and MD,7 finding increased BLB permeability in the symptomatic ears of patients with unilateral MD compared with their asymptomatic ears, which is in keeping with findings from animal studies of hydrops.8,9 Although BLB permeability in MD is a promising target for further exploration, it is not clear that these findings are specific for MD. Similar findings have been seen in patients with idiopathic sudden sensorineural hearing loss (ISSNHL),10 which can present similarly, and in a series of nonspecific sudden hearing loss.11
The purpose of this study is to compare MD with ISSNHL with respect to BLB permeability. Our hypothesis is that increased BLB permeability is a feature more strongly associated with MD than ISSNHL.
Materials and Methods
Patients
Institutional review board approval was obtained for creation of a prospective data base of patients imaged with delayed intravenous contrast-enhanced hydrops-protocol inner ear MR imaging, including a waiver of Health Insurance Portability and Accountability Act authorization and waiver of informed consent. Patients with ISSNHL or MD imaged between November 2012 and September 2013 were included in the study. Patients with bilateral disease and patients with unspecified laterality were excluded. All patients were followed clinically to establish a diagnosis based on the American Academy of Otolaryngology–Head and Neck Surgery criteria for ISSNHL and MD. A total of 84 patients were imaged, including 38 patients with definite MD and 12 with ISSNHL. The remaining patients (n = 34) did not have a clinical diagnosis of either condition and were excluded. Of the 50 patients with MD or ISSNHL, 5 patients with MD and 1 patient with ISSNHL were excluded because of bilateral disease or unspecified laterality of disease and 1 patient with MD was excluded because of history of endolymphatic shunt surgery. The final analysis group included 43 patients, 32 with MD (15 male, 17 female) and 11 with ISSNHL (8 male, 3 female), with an average age of 53.6 ± 13.4 years (range, 27–89 years). Demographic data are summarized in Table 1.
Patient demographics
Imaging Protocol
Imaging was performed on a 3T Magnetom Skyra unit (Siemens, Erlangen, Germany) by using a 16-channel head and neck coil 4 hours after an intravenous injection of 0.2 mmol/kg gadopentate dimeglumine (Magnevist; Bayer HealthCare Pharmaceuticals, Wayne, New Jersey). Scanning consisted of a “cisternographic” heavily T2-weighted 3D TSE sequence (sampling perfection with application-optimized contrasts by using different flip angle evolutions [T2 SPACE]; Siemens) and a heavily T2-weighted 3D FLAIR sequence. The T2 SPACE sequence was acquired with the following parameters: section thickness, 1 mm; TR/TE, 1430/265 ms; number of averages, 2; echo-train length, 98; flip angle, 140; matrix, 320 × 320; FOV, 200 × 200 mm. The heavily T2-weighted FLAIR sequence was acquired with the following parameters: section thickness, 0.8 mm; TR/TE, 9000/534 ms; inversion time, 2350 ms; number of averages, 2; echo-train length, 144; flip angle, 120; matrix, 320 × 260; FOV, 200 × 167 mm. All sequences were performed as high-resolution axial scans through the inner ear and internal auditory canals. The imaged volume included both inner ears.
The T2 SPACE sequence shows bright signal in both the endolymph and perilymph. The heavily T2-weighted FLAIR sequence shows bright signal only in the perilymphatic space because of diffusion of gadolinium into this compartment. The endolymphatic space remains low in signal on these sequences because of the impermeability of the tight junctions in the membranous labyrinth.
Measurement of Perilymph Signal as a Surrogate Marker of BLB Permeability
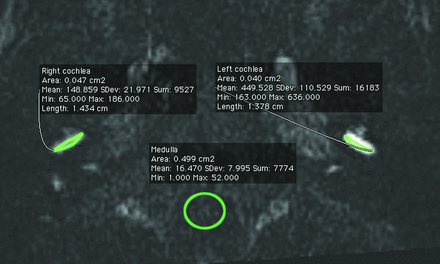
Increasing perilymph signal intensity on the heavily T2-weighted FLAIR sequence is related to increased BLB permeability,12,13 though the exact relationship has not been determined. In an animal model, the injection of lipopolysaccharide induced an increase in permeability as measured by iron oxide particle extravasation, which correlated with increased gadolinium enhancement in the perilymph.14 Perilymph signal was evaluated quantitatively. A postdoctoral research fellow with 3 years of experience in temporal bone imaging research (M.N.P.) performed all measurements, which were reviewed by a subspecialty-certified diagnostic neuroradiologist (A.R.S.) with 10 years of experience in MR imaging interpretation who interprets all hydrops-protocol MRIs at our institution. A freehand polygonal ROI was set manually in the basal turn of the cochlea on the heavily T2-weighted FLAIR image to include as much of the perilymph as possible, with size of approximately 5 mm2. A 50-mm2 circular ROI in the same plane as the cochlear basal turn was drawn in the medulla. The mean signal intensity was recorded for each ROI. The cochlea/medulla (CM) ratio was defined as the signal intensity of the basal turn divided by that of the medulla. The mean CM ratio of all affected and unaffected ears was calculated for all patients. An example ROI is shown in Fig 1.
3D-FLAIR MR imaging signal intensity measurements. Measurement of signal intensity was performed by drawing an elliptical ROI at the basal turn of each cochlea and a circular ROI at the medulla. The average intensity of each ROI was used to calculate the CM ratio.
Evaluation of Hydrops
Each ear was evaluated for the presence or absence of hydrops by using quantitative criteria. At the time of initial scan acquisition, the images were reconstructed as 3D maximum intensity projections. The neuroradiologist (A.R.S.), who was blind to the diagnosis and side of symptoms, outlined the endolymph and vestibule with freehand ROIs. The endolymph/vestibule ratio was calculated. Endolymph occupying >50% of the vestibule was graded as positive for hydrops, as per the criteria of Sepahdari et al.2
Statistical Analysis
Ears were segregated into 4 groups: symptomatic MD ears, asymptomatic ears in patients with MD, symptomatic ISSNHL ears, and asymptomatic ears in patients with ISSNHL. Descriptive statistics of mean and standard deviation were obtained. A least-squares difference method was used to compare groups; ANOVA was performed initially, with subsequent Student t test between groups. P < .05 was used as the threshold for statistical significance.
A mixed-effect model with a random intercept was used to test the differences in perilymph signal intensity between the affected and nonaffected ear and between the MD and ISSNHL groups after Box-Cox transformation for normality in the intensity. Patients were used as a random effect.
Receiver operating characteristic analysis was used to determine the performance of various CM ratios for identifying MD. For receiver operating characteristic analysis, the asymptomatic ear of patients with ISSNHL was set as a control value. This determination was based on the fact that there are no known or previously proposed MR imaging abnormalities in the asymptomatic ear of patients with ISSNHL.
The signal intensity ratios of symptomatic ears and asymptomatic ears in the same patients were also compared. Finally, the relationship between CM ratio and endolymphatic space dilation was assessed in a descriptive categoric fashion and quantitatively by using Spearman rank order correlation.
Results
CM Ratio in All Ears
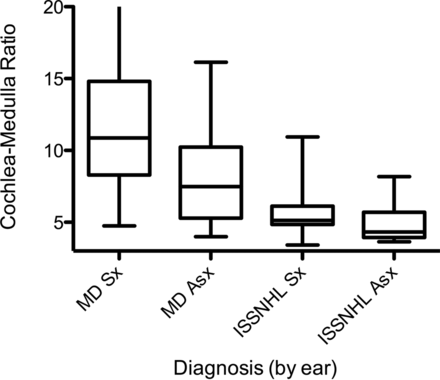
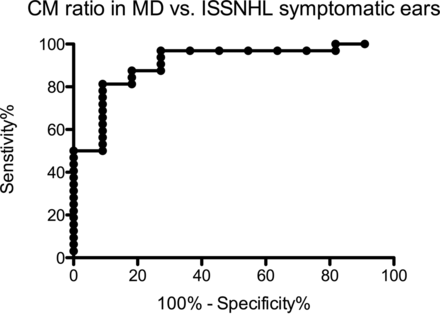
ANOVA showed significant differences across the 4 groups (F = 10.1, P < .0001). The results are shown graphically in Fig 2. Symptomatic MD ears showed the highest CM ratio (mean and standard deviation of 12.6 ± 7.4), indicative of the highest permeability. This was significantly higher than all other ears, with statistically significant differences compared with asymptomatic ears in patients with MD (8.0 ± 3.1, P = .0002), symptomatic ISSNHL ears (5.7 ± 2.0, P < .0001), and asymptomatic ears in patients with ISSNHL (5.0 ± 1.5, P < .0001). Among the 11 patients with ISSNHL, a paired t test revealed no significant difference between affected and unaffected sides. Receiver operating characteristic analysis for CM ratio demonstrated 0.91 area under the curve in differentiating MD from ISSNHL in the symptomatic ear (Fig 3). A CM ratio > 7.3 was 81% sensitive and 91% specific for MD. Other CM ratios and their associated sensitivities and specificities for MD are provided in Table 2.
CM ratio for all groups. Box plots show median and interquartile ranges, with bars depicting range. Note the significant increase in CM ratio among symptomatic MD ears when compared with all other ears and increased CM ratio in asymptomatic ears of patients with MD.
Receiver operating characteristic curve for CM ratio, comparing symptomatic MD ears to symptomatic ISSNHL ears. This curve demonstrates high discriminatory power of increased signal for MD. A CM ratio > 7.3 was 81% sensitive and 91% specific for MD.
CM ratios and associated performance for diagnosing MD compared with a control group of asymptomatic ears of patients with ISSNHL
Mean intensity of perilymph signal was significantly different between the symptomatic and nonsymptomatic ear (P < .001) and between the MD and ISSNHL group (P < .001) with 4.99 and 3.57 perilymph signal ratios, respectively. After adding an interaction between MD versus ISSNHL group and symptomatic versus nonsymptomatic in the model, the mean intensity was still significant between the clinical groups (MD versus ISSNHL, P < .001) and the interaction (P = .041). The mean intensity was 3.9 times higher for the symptomatic ear in the MD group compared with the intensity from all other ears.
CM Ratio Asymmetry in MD versus ISSNHL
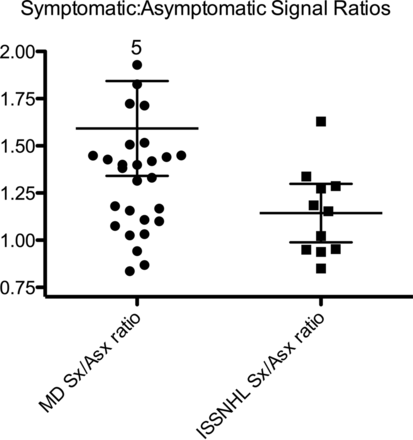
Patients with MD showed a greater degree of asymmetry of the affected ear to the unaffected ear compared with patients with ISSNHL. The symptomatic/asymptomatic CM ratio was 1.6 ± 0.7 in patients with MD, compared with 1.2 ± 0.2 in patients with ISSNHL (P = .04) (Fig 4). Although there was greater asymmetry between symptomatic and asymptomatic ears in MD compared with ISSNHL with respect to CM ratio, the effect was blunted by the often bilateral nature of CM ratio elevation in the setting of MD. As a result, the area under the receiver operating characteristic curve was only 0.75 when comparing MD to ISSNHL based on the degree of symptomatic/asymptomatic CM ratio asymmetry. A symptomatic/asymptomatic ratio of >1.4 was 50% sensitive and 90% specific in differentiating MD from ISSNHL.
Comparison of symptomatic and asymptomatic perilymph signal ratios between MD and ISSNHL. Patients with MD had a significantly greater asymmetry (P = .03), though 11 of 32 patients with MD showed virtually no asymmetry. Five patients had greater than double the perilymph signal intensity in the symptomatic ear.
CM Ratio in Asymptomatic Ears: Comparison of MD and ISSNHL
For asymptomatic ears, the mean CM ratio was higher in patients with MD than in patients with ISSNHL (8.0 ± 3.1 in patients with MD versus 5.0 ± 1.5 in patients with ISSNHL; P = .003) (Fig 2). Elevated CM ratio in both ears was seen in 1 patient with right-sided MD who subsequently developed symptoms in the left ear with confirmatory imaging evidence of hydrops (Fig 5). CM ratio also was higher in asymptomatic MD ears than in symptomatic ISSNHL ears (P = .02).
Initial and follow-up studies in a patient with unilateral right MD that progressed to bilateral disease. A and B, Initial MR imaging shows hydrops involving the right vestibule (short arrow) and cochlea (long arrow). There is increased perilymph signal intensity in both ears, but the left ear (B) is asymptomatic and shows no hydrops. The patient underwent right vestibular neurectomy, with improvement in symptoms. C and D, Follow-up study 18 months after A and B, performed because of new symptoms of aural fullness in the left ear, shows new hydrops involving the left vestibule (short arrow). Hydrops in the right vestibule has improved, though there is persistent hydrops in the right cochlea (long arrow).
Relationship between Hydrops, CM Ratio, and Diagnosis
Endolymph/vestibule ratio of >50% was present in 22 of 32 patients with MD for a sensitivity of 69%. This finding was 100% specific for symptomatic hydrops (ie, none of the asymptomatic ears in patients with MD and none of the 11 symptomatic or 11 asymptomatic ears in patients with a clinical diagnosis of ISSNHL showed hydrops). Of the 10 patients with a clinical diagnosis of MD but no MR evidence of hydrops, 5 had CM ratio > 10.8 in the symptomatic ear, which was greater than the maximum CM ratio of the ISSNHL group. There was no relationship between the CM ratio and the degree of hydrops as measured quantitatively from 3D maximum intensity projections (Spearman r = 0.08, P = .71).
Discussion
Increased postcontrast signal intensity in the cochlear basal turn has been shown to reflect BLB breakdown with associated increased contrast permeability.15,16 This permeability increase has been identified in patients with MD and in patients with ISSNHL,7,17 but these conditions have not previously been compared with each other in a quantitative manner with respect to this finding. Our results show that increased BLB permeability is a feature that is more clearly associated with MD than with ISSNHL. We also observed increased average permeability of asymptomatic ears of patients with MD compared with asymptomatic ears of patients with ISSNHL. Furthermore, in the subset of patients with MD without MR evidence of hydrops, 50% (5/10) showed markedly increased BLB permeability.
There are several implications of our results. First, these results could improve the ability to confirm a clinical suspicion of MD by using delayed intravenous contrast-enhanced 3D-FLAIR MR imaging. The actual diagnostic performance of this test and its interpretation are incompletely understood. Although some previous studies have reported 90% sensitivity of delayed intravenous contrast-enhanced 3D-FLAIR MR imaging for identifying ballooning of the endolymphatic system in the setting of MD,3,6 those results must be interpreted with caution. These studies did not include a sufficient control group of patients without MD, and they report hydrops in asymptomatic contralateral ears of patients with MD at a frequency of 22%–75%.3,6 Although it is certainly possible that hydrops may be present in asymptomatic ears of patients with MD, such findings could also reflect a systematic overcalling of hydrops. Our 69% sensitivity for hydrops in clinically involved MD ears is lower than other studies, but we did not observe hydrops in ears that were not clinically involved by MD. This may reflect more stringent criteria for calling hydrops and a lower rate of false-positives. Specifically, we do not identify hydrops if there is not involvement of the vestibule, as we find that evaluation of cochlear hydrops is inconsistent. It is clear that radiologic assessments of hydrops are sometimes discordant from the clinical diagnosis of MD, which itself can be controversial. The addition of imaging information related to increased BLB permeability in these patients could reduce this discrepancy.
A second implication of these results is that increased BLB permeability may be a biomarker of disease status in MD. This does not replace clinical evaluation, but could be complementary to clinical evaluation, particularly when assessing the effectiveness of various treatments. Clinical symptoms in MD are known to fluctuate or even burn out completely, and the clinical course does not always indicate whether an apparent response to treatment truly reflects modification of the disease process versus the natural course of the disease.18 Further follow-up of patients imaged with hydrops-protocol MR imaging is essential for answering these questions.
The finding of increased BLB permeability in asymptomatic ears of patients with MD also suggests that MD may be a systemic process that involves both ears to some degree. This is supported by clinical data showing that many patients with MD suffer bilateral disease, with onset of symptoms in each ear often occurring at different times.19 Although the true rate of bilateral involvement by MD is difficult to determine, it is undoubtedly sufficiently high as to eliminate the possibility that MD reflects an entirely random event localized to the inner ear. Anecdotally, we have clinically observed patients with bilateral disease who only have apparent hydrops in 1 ear. The current study was limited to patients with unilateral clinical disease, but future systematic analysis of patients with clinically bilateral disease may further clarify these issues.
Our data show a wide distribution of signal intensities in the asymptomatic ears of patients with MD. In contrast, we observed a tight distribution of signal intensities in asymptomatic ears of patients with ISSNHL. One patient with MD in our cohort showed increased permeability in both the symptomatic ear and asymptomatic contralateral ear and subsequently developed MD symptoms in the contralateral ear with imaging evidence of hydrops on a follow-up study (Fig 5). Although this was just a single case, it suggests that increased signal in the asymptomatic ear could predict progression to bilateral disease. Further follow-up of this cohort would help elucidate the relationship between changes in signal intensity and clinical course. In addition, studies on larger groups of patients would help increase the specificity of the CM ratio in differentiating MD from ISSNHL.
Limitations
The major limitation of this study is that it lacked a control group of asymptomatic healthy patients. The asymptomatic ear was used as an internal control in all cases, but systemic processes may affect BLB permeability in both ears and, therefore, may mask abnormalities in the symptomatic ears. A second limitation was the relatively small number of patients and relatively short follow-up period. We cannot exclude the possibility that some patients with apparent ISSNHL had incipient MD with monosymptomatic onset. This could produce factitious overlap between the MD and ISSNHL groups. Analysis of larger groups of patients, imaging of normal controls, and further clinical follow-up are necessary to confirm our findings and establish their significance. A third limitation is that imaging was obtained at only a single time point without obtaining precontrast 3D-FLAIR sequences or postcontrast sequences at multiple time points. In theory, increased signal on the 4-hour delay postcontrast 3D-FLAIR sequence could reflect a process other than increased permeability. For example, intrinsic precontrast hyperintensity can relate to proteinaceous signal contents, a phenomenon that has been noted in vestibular schwannoma20,21 and in ISSNHL.22 However, precontrast FLAIR hyperintensity has not been described in MD. Therefore, we think it is unlikely that the apparent BLB permeability increase in patients with MD in our study was influenced by precontrast asymmetries. Alternatively, increased basal turn perilymph signal could reflect impaired reabsorption of contrast from the inner ear or more inhomogeneous contrast distribution throughout the inner ear rather than excessive permeability. A final limitation is that the precise relationship between perilymph signal intensity and gadolinium concentration by using the heavily T2-weighted FLAIR pulse sequence is not known. It would be helpful to establish the mathematical relationship between perilymph signal and gadolinium concentration for this particular sequence and contrast agent so that the results could be applied to other centers.
Conclusions
We found that apparent BLB permeability was higher in MD than ISSNHL. This apparent permeability increase was seen in the absence of hydrops in some patients with clinical diagnoses of definite MD and in the asymptomatic ears of some patients with unilateral MD, suggesting a systemic abnormality in MD. BLB permeability may prove to be a biomarker of MD.
Footnotes
M.N. Pakdaman and G. Ishiyama contributed equally to this work.
Disclosures: Whitney B. Pope—UNRELATED: Consultancy: Celldex Therapeutics, Tocagen; Payment for Lectures (including service on Speakers Bureaus): Blue Earth Diagnostics.
Paper previously presented at: Annual Meeting of the American Society of Head and Neck Radiology, September 10–14, 2014; Seattle, Washington.
References
- Received December 17, 2015.
- Accepted after revision March 26, 2016.
- © 2016 by American Journal of Neuroradiology