Abstract
BACKGROUND AND PURPOSE: Acute C1–C2 fractures are difficult to detect on MR imaging due to a paucity of associated bone marrow edema. The purpose of this study was to determine the diagnostic utility of increased STIR signal in the posterior atlanto-occipital and atlantoaxial membrane complex (PAOAAM) in the detection of acute C1–C2 fractures on MR imaging.
MATERIALS AND METHODS: Eighty-seven patients with C1–C2 fractures, 87 with no fractures, and 87 with other cervical fractures with acute injury who had both CT and MR imaging within 24 hours were included. All MR images were reviewed by 2 neuroradiologists for the presence of increased STIR signal in the PAOAAM and interspinous ligaments at other cervical levels. Sensitivity and specificity of increased signal within the PAOAAM for the presence of a C1–C2 fracture were assessed.
RESULTS: Increased PAOAAM STIR signal was seen in 81/87 patients with C1–C2 fractures, 6/87 patients with no fractures, and 51/87 patients with other cervical fractures with 93.1% sensitivity versus those with no fractures, other cervical fractures, and all controls. Specificity was 93.1% versus those with no fractures, 41.4% versus those with other cervical fractures, and 67.2% versus all controls for the detection of acute C1–C2 fractures. Isolated increased PAOAAM STIR signal without increased signal in other cervical interspinous ligaments showed 89.7% sensitivity versus all controls. Specificity was 95.3% versus those with no fractures, 83.7% versus those with other cervical fractures, and 91.4% versus all controls.
CONCLUSIONS: Increased PAOAAM signal on STIR is a highly sensitive indicator of an acute C1–C2 fracture on MR imaging. Furthermore, increased PAOAAM STIR signal as an isolated finding is highly specific for the presence of a C1–C2 fracture, making it a useful sign on MR imaging when CT is either unavailable or the findings are equivocal.
ABBREVIATIONS:
- IDEAL
- iterative decomposition of water and fat with echo asymmetric and least squares estimation
- NF
- no fracture
- OF
- other cervical fracture
- PAOAAM
- posterior atlanto-occipital and atlantoaxial membrane complex
Injuries at the craniocervical junction occur in approximately 30% of patients presenting with blunt cervical spine trauma resulting in osseous and/or ligamentous injury.1,2 Given the high potential for neurologic morbidity associated with these injuries, accurate and timely assessment is critical for improved patient outcomes.3,4 Due to its high sensitivity for detecting acute fractures or dislocations by virtue of its high spatial resolution, ability to obtain multiplanar reformations, and speed, multidetector CT is the established initial imaging technique in patients suspected of having craniocervical and other cervical spine injuries.5⇓–7 Although MR imaging has superior ability for the evaluation of soft-tissue and spinal cord injuries and the determination of fracture acuity, its use is generally limited to cases with evidence of severe injuries on CT, abnormal neurologic findings, or equivocal CT findings. This secondary role of MR imaging in screening cervical spine injuries is driven not only by its higher cost, lower speed, and decreased availability compared with CT, but also by its decreased sensitivity for craniocervical junction and posterior element fractures.8,9
Recent work has shown that type II odontoid fractures in older patients may not exhibit STIR hyperintense marrow signal at the fracture site, limiting the utility of MR imaging in the evaluation of the presence and acuity of a fracture.10,11 Nevertheless, because of the potential for concomitant ligamentous and neurologic injuries associated with craniocervical fractures, MR imaging is frequently used as a complementary technique to CT at many tertiary care centers.12,13 In some of these instances, the initial CT or report obtained at an outside hospital may not be available for review by the radiologist interpreting follow-up MR imaging in a timely fashion, or CT may have equivocal findings, creating a perfect scenario for missing C1–C2 fractures on MR imaging, with the potential for medicolegal implications.
We have observed increased signal on STIR images in the region of the posterior atlanto-occipital and atlantoaxial membranes, considered in the current study as a single complex (PAOAAM), in patients with acute C1–C2 fractures. However, this finding is typically not seen in patients presenting with trauma without cervical fractures, leading us to hypothesize that this may be a useful diagnostic indicator of acute C1–C2 fracture on MR imaging. Based on this hypothesis, the purpose of the study was to determine the diagnostic utility of increased PAOAAM STIR signal in the detection of acute C1–C2 fractures, the presence of which may prompt the reader to repeat or reinterpret CT cervical spine studies with equivocal findings.
Materials and Methods
Patients and Control Groups
Following institutional review board approval for this Health Insurance Portability and Accountability Act–compliant study, a retrospective review from 2008 to 2015 of our institutional imaging data base was performed for patients with acute isolated C1–C2 fractures who had both CT and MR imaging of the cervical spine within 24 hours of each other. Per review of the medical records, the CT examinations were performed within 24 hours of trauma. CT performed at an outside hospital before transfer was included if it had been performed within 24 hours. All MR images were obtained at our institution. The presence of acute C1–C2 fractures was determined by the CT report and history/clinical examination documented in the medical records. One hundred two patients with C1–C2 fractures were identified. Nine fractures were determined to be chronic on the basis of review of prior imaging and/or clinical history and were excluded from analysis. Six additional patients were excluded due to the absence of diagnostic STIR images, leaving 87 patients.
Two control groups with CT scans obtained within 24 hours of blunt cervical spine trauma and MR images within 24 hours of the CT were selected consecutively by reviewing the imaging data base from 2013 to 2015 until each group included 87 patients. The controls consisted of a no fracture (NF) group and an other fracture (OF) group. The NF group included patients with a clinical history of blunt cervical spine trauma without reported fractures on CT. The OF group included all fractures isolated in the cervical spine (regardless of fracture morphology), excluding concomitant C1–C2 fractures.
CT and MR Imaging
Noncontrast CT of the cervical spine was performed on a 128–detector CT scanner (Discovery HD750; GE Healthcare, Milwaukee, Wisconsin). Images were acquired helically with a section thickness of 2.5 mm, a pitch of 0.984, a gantry rotation time of 1 second, at 120 kV with a tube current of 340 mA. All outside CT scans were also helically acquired and obtained on 64– or 128–detector CT scanners.
All MR imaging examinations were performed on one of two 1.5T MR imaging scanners with our standard departmental protocol of T1- and T2-weighted and STIR or STIR-equivalent iterative decomposition of water and fat with echo asymmetric and least squares estimation (IDEAL) sagittal water-selective images and axial T2-weighted and gradient recalled-echo images. On the Signa HDx scanner (GE Healthcare), STIR (2008–2012) imaging parameters were TR/TE = 3750/60 ms and TI = 150 ms, and IDEAL (2012–2015) imaging parameters were TR/TE = 4222/85 ms. For both sequences, the matrix was 320 × 256. For the Magnetom Espree (Siemens, Erlangen, Germany) scanner, the parameters were TR/TE = 5570/64 ms, TI = 150 ms, and matrix = 320 × 224. For both GE Healthcare and Siemens scanners, the FOV was 220 mm and the section thickness was 3 mm.
Image Analysis
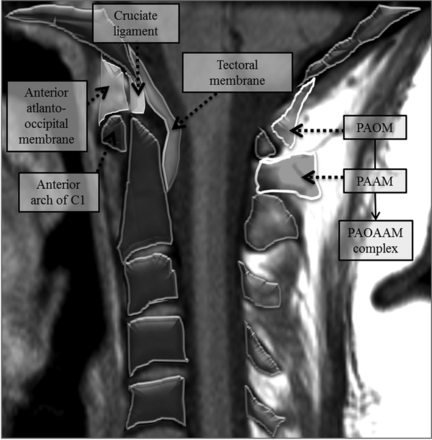
All CT scans obtained in our institution were interpreted by a board-certified radiologist (emergency department radiologist or neuroradiologist). The subspecialty training of outside institution interpreters was not known. The CT studies were not independently re-reviewed by the investigators. For simplicity, STIR and IDEAL images will be collectively referred to as STIR images. STIR images from MR images were independently reviewed by 2 board-certified, fellowship-trained neuroradiologists (with 25 and 2 years of postfellowship experience), blinded to the CT reports and images, for the presence of increased signal on STIR images in the region of the PAOAAM. The increased STIR signal in the PAOAAM was defined as increased signal in one or both of the posterior atlanto-occipital and the posterior atlantoaxial membranes, which are considered in the current study as a single complex (PAOAAM) (Fig 1). STIR images were also used to assess the presence of increased signal in interspinous ligaments at other cervical levels (Figs 1⇓⇓–4).
Overview of the anatomy at the craniocervical junction. The posterior atlanto-occipital membrane (PAOM) is a thickened band of the ligamentum flavum extending from the posterior arch of the atlas to the posterior occipital bone. The posterior atlanto axial membrane (PAAM) is a correlate extending from the posterior arch of the atlas to the posterior elements of C2. The PAOM and PAAM are considered a single complex in this article (PAOAAM).
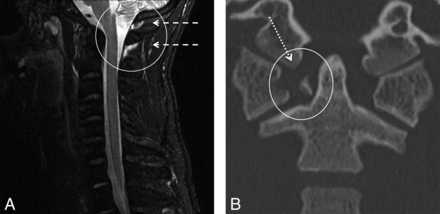
Isolated increased PAOAAM signal. A, STIR midline sagittal image of a 26-year-old man, presenting after assault. There is an isolated increased STIR signal (circle and dashed arrows) at the PAOAAM and none at the interspinous ligaments. B, CT demonstrates the anterior and posterior arches of C1 fractures, with an avulsed transverse ligament (circle and dotted arrow) and lateral subluxation of the lateral masses.
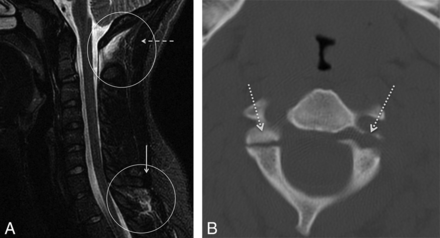
Increased PAOAAM signal. A, STIR midline sagittal image of a 17-year-old adolescent girl, presenting after a motor vehicle collision. There is an increased STIR signal at the PAOAAM (circle and dashed arrow) and at the interspinous ligaments (circle and solid arrow). B, CT demonstrates comminuted fractures of the bilateral C2 pedicles (dotted arrows).
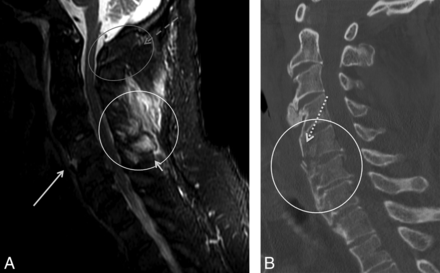
Other interspinous ligament signal. A, STIR midline sagittal image of a 66-year-old man presenting after a fall secondary to intoxication. There is no STIR signal at the PAOAAM (gray circle and dashed arrow), but only at the interspinous ligaments (white circle and short solid arrow). There is an interruption at the anterior longitudinal ligament at C5–6 (long solid arrow). B, CT demonstrates a fracture of the ossified anterior longitudinal ligament at C5–6 (circle and dotted arrow).
Data Analysis
Differences in age among C1–C2, NF, and OF groups were assessed by using 1-way ANOVA. Interobserver reliability for both increased PAOAAM and interspinous ligament STIR signal was measured with κ coefficients. Disagreements were resolved by consensus agreement between the 2 interpreters, and the reconciled data were used for subsequent analyses. The frequency of increased STIR signal in the PAOAAM and the presence of increased STIR signal in the interspinous ligaments at other cervical levels were determined for all patients. The sensitivity and specificity of increased STIR signal in the PAOAAM, both isolated to the PAOAAM and in the presence of STIR hyperintensity at other cervical levels, were assessed for the C1–C2, NF + OF (all controls), NF, and OF groups. We also assessed the sensitivity and specificity of isolated increased STIR signal in the PAOAAM associated with C1–C2 fractures by comparing these groups. Subgroup analysis of C1–C2 fracture types separated into odontoid type II, odontoid type III, C2 pars interarticular (hangman), C2 lamina or transverse process, C2 comminuted vertebral body, isolated C1, and combined C1 and C2 fractures with presence of increased STIR signal in the PAOAAM was performed with the χ2 test. Additional analysis of patients with NF separated into midcervical (C3 through C4), lower cervical (C5 through C7), and mixed (C3 through C4 and C5 through C7) level fractures with presence of increased STIR signal in the PAOAAM was also performed. Statistical significance was set at P ≤ .05.
Results
Demographic characteristics of patients with C1–C2 fractures, NF, and OF are shown in Table 1. There were no significant age differences among the C1–C2 fracture, NF, and OF groups (P ≤ .2, Table 1). With terminology established by Landis and Koch,14 there was “almost perfect” interobserver agreement between readers in the detection of PAOAAM signal (κ = 0.91; 95% CI, 0.88–0.97) and interspinous ligament signal at other cervical levels (κ = 0.93; 95% CI, 0.89–0.98).
Patient characteristics: sex distribution
The 87 C1–C2 fractures were the following: odontoid type II (n = 27), odontoid type III (n = 19), C2 pars interarticular (hangman) (n = 7), C2 lamina or transverse process fractures (n = 3), C2 comminuted vertebral body (n = 8), isolated C1 (n = 20), and combined C1 and C2 (n = 3). Twenty-five of 27 odontoid type II, 18/19 odontoid type III, 7/7 hangman, 3/3 C2 lamina or transverse process, 8/8 C2 comminuted vertebral body, 19/20 isolated C1, and 3/3 combined C1 and C2 fractures demonstrated increased PAOAAM STIR signal. No significant difference was found in the presence of PAOAAM signal among the C1–C2 fracture groups (χ2 = 1.52, P = .96).
Fractures at other cervical levels included 16 mid- (C3 through C4), 59 lower- (C5 through C7), and 12 mixed-level fractures. Nine of 16 mid-, 33/59 lower-, and 9/12 mixed-level fractures were associated with increased PAOAAM STIR signal. No significant difference in the presence of PAOAAM signal among fractures involving mid-versus-lower cervical spine was noted (χ2 = 1.54, P = .46).
Two patterns of increased STIR signal were observed in patients with acute C1–C2 fractures: 1) increased STIR signal in the PAOAAM with increased STIR signal in ligaments at other cervical levels (Fig 3), and 2) isolated increased STIR signal localized to the PAOAAM without increased signal in ligaments at other cervical levels (Fig 2).
Increased STIR signal in the PAOAAM was seen in 81/87 (93.1%) patients with C1–C2 fractures, 6/87 (6.9%) with NFs, and 51/87 (58.6%) with OFs. The sensitivity and specificity of increased STIR signal in the PAOAAM in detecting acute fractures at C1–C2 are shown in Table 2. Isolated increased STIR signal in the PAOAAM was seen in 52/87 (59.8%) patients with C1–C2 fractures, 4/87 (4.6%) with NFs, and 7/87 (8.0%) with OFs. The sensitivity and specificity of isolated increased STIR signal in the PAOAAM in detecting acute fractures at C1–C2 are shown in Table 3.
Diagnostic utility of increased stir signala at the PAOAAM in detecting the presence of an acute C1–C2 fracture
Diagnostic utility of isolated increased STIR signala at the PAOAAM in detecting the presence of an acute C1–C2 fracture
Discussion
Our results show that increased STIR signal is observed in most patients with C1–C2 fractures and that this finding is a highly sensitive MR imaging sign of fractures in this area. We also observed that this finding has high specificity when compared with patients without cervical spine fracture (patients with NF). However, the specificity decreases when compared with patients with other cervical fractures (OF group) and all controls (NF + OF). This decreased specificity in relation to all controls and the OF group was due to the presence of increased STIR signal in the PAOAAM in many patients with other cervical spine fractures, suggesting that this structure is frequently affected in any type of cervical spine fracture. We also observed that when increased PAOAAM STIR signal is an isolated finding in patients with cervical spine injury and there is no associated increased signal in ligaments at other cervical levels, this finding has high specificity in indicating the presence of a C1–C2 fracture. On the basis of our observations, we believe that increased STIR signal in the PAOAAM should make a radiologist suspicious of the presence of a cervical spine fracture when interpreting a cervical spine MR imaging study. Furthermore, when the increased STIR signal in the PAOAAM is an isolated finding, a C1–C2 fracture should be suspected until proved otherwise by CT.
The STIR sequence on MR imaging is known to be very sensitive for the detection of a marrow edema pattern in subtle compression or micro-/insufficiency fractures, particularly of the thoracic and lumbar spines and appendicular skeleton, such as the pelvis and femur.15⇓⇓–18 Conversely, in the cervical spine, prior work has shown not only the superiority of CT in the detection of bony injuries, but also that STIR imaging is much less sensitive for the detection of acute fractures. Holmes et al,9 showed that of 66 patients with both CT and MR imaging examinations, the sensitivity for osseous fractures was 95% on CT versus 50% on MR imaging, with most of the missed lesions involving the lateral and posterior elements, with the caveat that the specific time interval between the CT and MR imaging studies was not reported by the authors.
Limitations of the STIR sequence were first demonstrated by Peri et al in 2009 in type II and III odontoid fractures in which STIR abnormalities were not seen in 22% of acute fractures and were limited to the fracture cleft in 11/18 patients.10 In addition, a more recent study reported that the sensitivity of STIR signal for demonstrating acute type II odontoid fractures was only 82% in patients younger than 57 years of age and became significantly lower at 54% in patients older than 57 years of age.11 The mechanism underlying this difference in the sensitivity of MR imaging for the detection of acute fractures in the cervical spine versus the thoracic and lumbar spine is unclear; however, Lensing et al11 postulated that this issue may be due to a combination of progressive decreased vascularity at the odontoid base and age-related osteopenia.
Our findings also suggest that increased PAOAAM signal associated with C1–C2 fractures is not related to fracture morphology. Of note, prior work in the thoracolumbar spine reports that interspinous ligamentous injuries associated with fracture are correlated with the degree of kyphotic angulation and similar measures rather than fracture type.19,20 It is likely that the lack of correlation between C1–C2 fracture types and PAOAAM signal is due to the understanding that most cervical fractures are associated with high energy trauma, secondary to extreme flexion, extension, shearing, and rotation forces. Therefore, we believe that the findings outlined on our study can be generalized to any C1–C2 cervical fracture.21 In further support, cervical trauma associated with whiplash injury, presumably resulting from lower energy trauma than that associated with fractures, is not associated with PAOAAM injury compared with controls.22
The study has several limitations. First, we only looked at the STIR hyperintense signal of the PAOAAM and interspinous ligaments at other cervical levels and did not assess the marrow edema pattern or other soft-tissue signal in the current study. Although we specifically chose not to assess the marrow edema pattern in this study given the relatively lower sensitivity for odontoid and other craniocervical fractures on previous reports, such findings parenthetically noted may have resulted in some confirmation bias. Second, given that the OF and NF control groups were chosen consecutively among patients and not randomly, this method may have resulted in unforeseen selection bias. Third, because this report was focused on C1–C2 fractures, we did not analyze whether there were additional fracture types at the level of the craniocervical junction or skull base that may be associated with increased PAOAAM STIR signal. This omission could potentially result in lowered specificity of the findings for C1–C2 fractures. Further work elucidating this question will be required. We also did not independently review CT scans to confirm a diagnosis of cervical spine fracture, potentially resulting in over-/underestimation of the sensitivity and specificity of PAOAAM STIR signal for C1–C2 fractures. Finally, diagnosis of the acuity of the fracture was based on history/clinical findings, and we did not obtain a follow-up MR imaging to confirm whether and when PAOAAM increased signal subsequently resolved.
In our current study, we anecdotally noted 9 patients with a questionable history of prior C2 fracture whose cervical CT findings at the time of the new trauma were equivocal and later were determined to be chronic on the basis of prior imaging and/or clinical history and, therefore, were not included in the study. In these patients, the MR imaging performed within 24 hours of cervical CT did not demonstrate increased PAOAAM signal. Due to the small number of patients in this group, no definite conclusions could be drawn from this observation. Nevertheless, this finding suggests that increased signal at the PAOAAM is correlated with acute injury and is unlikely to be confounded by superimposed subacute-to-chronic injury. However, further study is required to address this issue. Furthermore, 6/87 patients in the C1–C2 fracture group did not demonstrate associated increased PAOAAM signal. The mechanism underlying this difference is uncertain and likely multifactorial, including mechanism of injury, cervical alignment, and bone density. For example, this subset of patients all demonstrated subjective decreased bone mineralization on CT examinations, though this was not confirmed by bone density examinations in the available medical records. Thus, it is possible that C1–C2 fractures in this false-negative group may occur with lower energy trauma and are less likely to result in ligamentous injury. Correlation with bone density in future studies would help elucidate these findings.
Conclusions
The presence of an increased PAOAAM signal on STIR images is a highly sensitive indicator of an acute C1–C2 fracture on MR imaging. As an isolated finding, increased PAOAAM signal is highly specific for the presence of a C1–C2 fracture, making it a diagnostically useful imaging sign for possible re-interpretation of reportedly negative/equivocal CT findings or repeat CT imaging if not available.
Footnotes
Disclosures: Rafael Rojas—UNRELATED: Consultancy: Guerbet, Comments: Guerbet MRI Advisory Board meeting, Chicago, July 2016.
References
- Received October 30, 2016.
- Accepted after revision May 4, 2017.
- © 2017 by American Journal of Neuroradiology