Abstract
BACKGROUND AND PURPOSE: The effect of exposing the developing brain of a high school football player to subconcussive impacts during a single season is unknown. The purpose of this pilot study was to use diffusion tensor imaging to assess white matter changes during a single high school football season, and to correlate these changes with impacts measured by helmet accelerometer data and neurocognitive test scores collected during the same period.
MATERIALS AND METHODS: Seventeen male athletes (mean age, 16 ± 0.73 years) underwent MR imaging before and after the season. Changes in fractional anisotropy across the white matter skeleton were assessed with Tract-Based Spatial Statistics and ROI analysis.
RESULTS: The mean number of impacts over a 10-g threshold sustained was 414 ± 291. Voxelwise analysis failed to show significant changes in fractional anisotropy across the season or a correlation with impact frequency, after correcting for multiple comparisons. ROI analysis showed significant (P < .05, corrected) decreases in fractional anisotropy in the fornix-stria terminalis and cingulum hippocampus, which were related to impact frequency. The effects were strongest in the fornix-stria terminalis, where decreases in fractional anisotropy correlated with worsening visual memory.
CONCLUSIONS: Our findings suggest that subclinical neurotrauma related to participation in American football may result in white matter injury and that alterations in white matter tracts within the limbic system may be detectable after only 1 season of play at the high school level.
ABBREVIATIONS:
- CTE
- chronic traumatic encephalopathy
- FA
- fractional anisotropy
- FXST
- fornix-stria terminalis
- TBSS
- Tract-Based Spatial Statistics
The association between head injury and neurodegeneration in contact sport athletes has been well established. Multiple postmortem case reports of former American football players with this neurodegenerative process, termed “chronic traumatic encephalopathy” (CTE), are present in the literature.1,2 Although most available data are from middle-aged or elderly individuals, there is a troubling report of early CTE pathologic changes in a deceased high school football player.3 CTE is believed to be related to repetitive brain injuries accrued across time, and the long-term consequences of exposing the developing brain of a high school football athlete to several hundred head impacts during a season have yet to be determined. With >1 million high school athletes playing football, there is a commensurately large need to elucidate the neurologic consequences of repetitive, subconcussive impacts.4
There is a growing body of evidence that diffusion tensor imaging can detect injuries to the white matter tracts related to contact sports exposure. One study, for example, found alterations in DTI measures of mean diffusivity and fractional anisotropy (FA) in high school football athletes that differed from those in controls.5 Most interesting, these alterations in DTI metrics were even more extensive in the single concussed subject in that study. These findings are corroborated by studies in which a greater risk-weighted head impact exposure index was associated with a greater number of voxels with altered FA.6,7 Expanding to other sports, the burden of heading events in adult soccer athletes has been associated with alterations in white matter diffusion metrics and diminished performance on computerized cognitive assessment.8,9
Given the evidence linking subconcussive impacts to neurophysiological and neuroanatomic alterations, the purpose of this pilot study was to use diffusion tensor imaging to assess white matter changes during a single season of high school football. Furthermore, we sought to correlate imaging data with helmet accelerometer and neurocognitive data collected during the same period. We hypothesized that alterations in white matter diffusion tensor metrics from preseason to postseason would correlate with cumulative subconcussive impacts, as measured by helmet accelerometers, and that these DTI changes would also correlate with alterations in neurocognitive measures.
Materials and Methods
Subjects
This was a prospective, longitudinal pilot study to investigate the effects of cumulative head impacts during 1 high school football season. An initial MR imaging study was performed for each subject before the start of fall contact practice (average, 4.6 ± 5.0 days before the first contact practice). Within 4 weeks of the end of the season (average, 14.5 ± 8.5 days after the final game), the subjects returned for the postseason MR imaging using the same protocol. If a subject was injured and missed a portion of the season, they were scanned after the completion of the team's season. Any subjects who experienced a diagnosed concussion were asked to undergo an additional MR imaging within 48 hours of the injury in addition to the pre- and postseason scans.
Institutional review board approval was obtained at both participating institutions, Duke University and University of North Carolina, Chapel Hill, before enrollment. Subjects were recruited from a local high school football team by an informational meeting held with players and parents following a noncontact preseason practice. Because all the subjects were minors, informed consent was obtained from the subject's legal guardian with assent from the subject before enrollment in the research study.
Any participant with a contraindication to MR imaging was excluded. Individuals with metallic dental or surgical implants that would excessively degrade the echo-planar images were also excluded. Any subject with a structural abnormality on MR imaging, including but not limited to tumors, hematomas, or intraparenchymal hemorrhages, was also excluded.
Neurocognitive Data
Before the start of the season and after the last game of the season, the subjects were administered a computer-based neurocognitive assessment using CNS Vital Signs (http://cnsvs.com/). The validity and reliability of CNS Vital Signs have been previously described.10,11 Main outcome measures included standard scores for the following domains: verbal memory, visual memory, psychomotor speed, cognitive flexibility, complex attention, processing speed, reasoning, reaction time, and executive functioning. Standard scores are based on a normative dataset that matches participants by age. Subjects also underwent assessment with Neuropsychological Assessment Metrics, which contained scores of physical and verbal aggression, anger, and hostility; aggressiveness, the Buss-Perry Aggression Questionnaire; anxiety, the State-Trait Anxiety Inventory; and impulsivity, the Barratt Impulsiveness Scale.12⇓–14
Accelerometer Data
Head impact biomechanics were measured using the Head Impact Telemetry System (Simbex; Lebanon, New Hampshire) mounted in the helmets, which recorded the incidence, direction, and severity of head impacts received by the players. The subjects wore the accelerometer-fitted helmets in all contact practices and games. Head impacts exceeding 10 g of linear acceleration were collected via radiofrequency communication to a sideline unit. Head Impact Telemetry System data were preprocessed by time-gating practice and game sessions and removing nonimpact artifacts.
MR Imaging
All pre- and postseason MR imaging was performed at the Brain Imaging and Analysis Center at Duke University Hospital on a 3T scanner (MR750; GE Healthcare, Milwaukee, Wisconsin) with an 8-channel head coil. Diffusion tensor imaging was acquired using the following parameters: b-value = 1000 s/mm2, 31 directions, TR/TE = 10,000/96 ms, FOV = 256 × 256 mm2, matrix size = 128 × 128, section thickness = 2 mm, 71 axial sections with no interslice gap, resolution = 2 × 2 × 2 mm3. 3D sagittal T1-weighted spoiled gradient-recalled echo was performed for anatomic reference (TE = 1.9 ms, TI = 400 ms, flip angle = 11°, voxel size = 0.93 × 0.93 × 1.2 mm3).
Image Preprocessing
Preprocessing was performed with the FMRIB Diffusion Toolbox (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FDT) to remove eddy current distortions and to correct for simple head motion. Eddy current distortion was corrected using the FSL software tool, eddy (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/eddy). It simultaneously models the effects of diffusion eddy currents and subject movements on the image. After preprocessing, all images were visually inspected for quality assurance.
Fractional anisotropy was chosen as the single DTI metric to be analyzed in this study for 2 primary reasons: First, FA is the most commonly studied metric in traumatic brain injury, allowing the results to be considered in the context of other available data. Second, given the small sample size and large number of regions studied, FA was chosen as the focus of the study to limit errors due to multiple comparisons. FA is a scalar value describing the degree to which diffusion is asymmetric, or anisotropic, and reflects the presence of underlying ordered microstructure within the tissue, such intact axons and/or myelin sheaths.15
Fractional anisotropy was calculated for each voxel, and the data were projected onto a group white matter skeleton using Tract-Based Spatial Statistics (TBSS; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS)16in the following manner: The subjects in the sample were coregistered using a method that ensured white matter alignment using an intermediate df and nonlinear registration to a 1 × 1 × 1 mm template (FMRIB58_FA; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FMRIB58_FA). Mean FA images were generated by averaging these normalized resampled images, and a mean FA skeleton was created in TBSS. After thresholding the skeleton to exclude low FA values (<0.2), we projected each subject's aligned FA image onto the mean FA skeleton.
TBSS Analysis
White matter skeleton difference images of postseason minus preseason were created to assess changes in FA across the season using a 1-sample t test. The difference images were also used to model the mean-centered, continuous covariate of the number of impacts across the season. Design matrices were created using the FSL General Linear Model tool (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/GLM), and the FSL Randomize tool (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Randomise/UserGuide)17 was used to perform nonparametric, permutation-based statistical inference with threshold-free cluster enhancement and correction for multiple comparisons.18 These methods have been described in detail elsewhere.16 T-statistics were used to determine main or interaction effects with an a priori α of .05 and 5000 conditional Monte Carlo permutations (giving a confidence limit of .05 ± .0062 for the P value).
TBSS ROI Analysis
Mean FA values were derived from the WM skeleton for 24 ROIs using the ENIGMA DTI Protocol (http://enigma.ini.usc.edu/protocols/dti-protocols/#eDTI) and applying the ICBM-JHU-DTI-81 whitematter atlas (http://neuro.debian.net/pkgs/fsl-jhu-dti-whitematter-atlas.html).19 Pre- to postseason differences in these mean FA values by region were modeled using a mixed linear model with region, number of impacts, and their interaction as fixed effects and subject as a random effect, as depicted in the following equation:
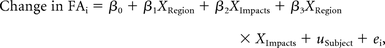 where β0 represents the intercept (mean FA change across all regions at the mean number of impacts); β1 though β3, the coefficients for region, impact number, and their interaction, respectively; u, a subject-specific random effect; and ei, the residual error term for an arbitrary measurement i. We chose this model to take into account the variability in individual subject responses, recognizing that repeat measurements across regions in the same subject are likely to be more similar to each other than to measurements from different subjects.20 Parameter estimates for main effects and interactions in each region were determined, with corresponding F-tests. Correlation of FA changes with neuropsychological measures was performed for regions showing a significant interaction. Statistical analyses were performed with R statistical software (http://www.r-project.org/),21 the statistical package nlme (https://cran.r-project.org/web/packages/nlme/index.html),22 SAS 9.3 (SAS Institute, Cary, North Carolina), and JMP 11.2.0 (SAS Institute).
where β0 represents the intercept (mean FA change across all regions at the mean number of impacts); β1 though β3, the coefficients for region, impact number, and their interaction, respectively; u, a subject-specific random effect; and ei, the residual error term for an arbitrary measurement i. We chose this model to take into account the variability in individual subject responses, recognizing that repeat measurements across regions in the same subject are likely to be more similar to each other than to measurements from different subjects.20 Parameter estimates for main effects and interactions in each region were determined, with corresponding F-tests. Correlation of FA changes with neuropsychological measures was performed for regions showing a significant interaction. Statistical analyses were performed with R statistical software (http://www.r-project.org/),21 the statistical package nlme (https://cran.r-project.org/web/packages/nlme/index.html),22 SAS 9.3 (SAS Institute, Cary, North Carolina), and JMP 11.2.0 (SAS Institute).
Results
Subjects
Our cohort consisted of 17 male athletes with a mean age of 16 ± 0.73 years and mean football experience of 7.8 ± 2.53 years. Six athletes reported at least 1 previous concussion using a standard definition of concussion23; of these 6, five subjects reported 3 previous concussions and 1 subject had 1 prior concussion. The mean number of impacts sustained across the season was 414 ± 291, with mean cumulative linear and rotational sums of 12,338 ± 9274 g and 836,367 ± 635,830 rad/s2, respectively. Eleven subjects played an offensive primary position, and the rest played defense primarily. Three subjects were linemen, and the remainder were skill positions (any other position except punter or kicker). Subject demographics and subconcussive impact burdens are presented in Table 1, sorted in descending order by the number of impacts. One subject (data listed by footnote a) was concussed on day 93 of the study. He underwent MR imaging the following day, in addition to his postseason scan 39 days after the injury; data from the concussed subject's pre- and postseason scans are included in all group analyses.
Demographic information and subconcussive head impact burden for study participantsa
TBSS Analysis
No clusters demonstrated a change in FA across the season that was significantly different from zero, after correcting for multiple comparisons. Similarly, no clusters demonstrated a linear relationship between a change in FA across the season and the number of impacts during the season.
TBSS ROI Analysis
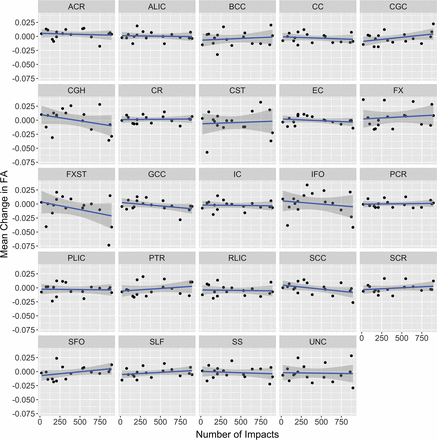
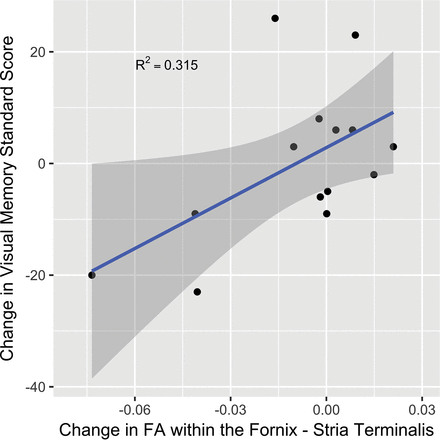
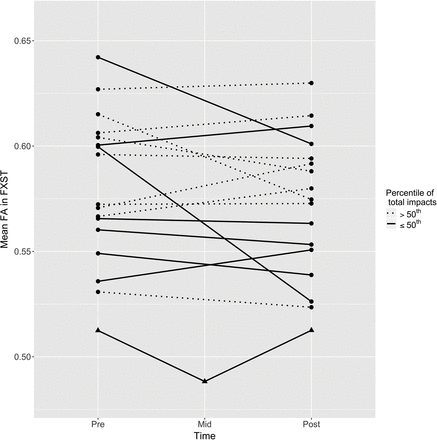
Parameter estimates of fixed effects for region, number of impacts, and their interaction terms are provided in Table 2. For the overall mean of the regions, there was no significant main effect across the season or interaction with impacts. The change in FA for each region is plotted as a function of the number of impacts in Fig 1. Of the specific regions examined, 2 were observed to have an interaction with the number of impacts: the cingulum hippocampus and the fornix-stria terminalis (FXST). The parameter estimates for these 2 interactions were both negative, indicating a negative change in FA with a higher number of impacts. Of these 2 regions, the FXST demonstrated a greater magnitude of interaction and a significant main effect, indicating an overall loss of FA at the mean number of impacts across all players. FA reduction in the FXST correlated with a decline in the visual memory score during the season, but not with other available CNS Vital Signs or Neuropsychological Assessment data. Pre- and postseason visual memory scores were available in 14 of the 17 players (Fig 2). Regarding the subject who was concussed during the season, FA in the FXST demonstrated a 4.7% reduction within 24 hours of the concussion. The FA value returned to its baseline level at his postseason follow-up scan (Fig 3).
Parameter estimates from a mixed linear modela
Mean change in FA for each ROI against the frequency of impacts across the season. Ninety-five percent confidence bands are presented as shadows around the linear regression line. CGH indicates cingulum hippocampus; CGC, cingulate gyrus; ACR, anterior corona radiata; ALIC, anterior limb of internal capsule; BCC, body of corpus callosum; CC, corpus callosum; CR, corona radiata; CST, corticospinal tract; EC, external capsule; FX, fornix; IC, internal capsule; GCC, genu of corpus callosum; IFO, inferior fronto-occipital fasciculus; PCR, posterior corona radiata; PLIC, posterior limb of internal capsule; PTR, posterior thalamic radiation; RLIC, retrolenticular part of internal capsule; SCC, splenium of corpus callosum; SCR, superior corona radiata; SFO, superior fronto-occipital fasciculus; SLF, superior longitudinal fasciculus; SS, sagittal stratum; UNC, uncinate fasciculus.
Scatterplot of changes in FXST FA versus change in the visual memory domain standard score from CNS Vital Signs. The 95% confidence band is presented as a shadow around the linear regression line.
Scatterplot of changes in FXST FA for all subjects across the season. Individual player data are connected by solid or dotted lines based on the median split of the total number of impacts during the season, as depicted in the legend. The concussed subject is plotted with triangles.
Discussion
In this pilot study, we used diffusion tensor imaging to examine the effects of subconcussive head trauma in high school football athletes during 1 season. We examined multiple white matter tracts across the brain for changes in FA as a function of the number of linear impacts exceeding a minimal threshold and observed decreases in fractional anisotropy with an increasing number of impacts in 2 tracts, the FXST and the cingulum hippocampus, both of which are components of the limbic system. Overall, the observed effects were greatest in the FXST, where there was also a significant main effect, meaning a fixed pre- to postseason decline in FA in the FXST, compared with other regions.
These results suggest that alterations in white matter tracts within the limbic system may be detectable after only 1 season of play at the high school level. Comparison of limbic system white matter changes with the CNS Vital Signs data revealed a correlation between change in FA in the FXST and visual memory; a reduction in FA corresponded to a worsening of visual memory scores. This same association of FA alterations in the fornix-hippocampal pathway and declining verbal memory scores has previously been found in concussed individuals, suggesting that neural networks involving memory are susceptible to injury and that these alterations in our subjects might be related to repetitive neurotrauma.24
The ROI-labeled fornix-stria terminalis (FXST) correlates anatomically with the crus of the fornix and stria terminalis, which cannot be individually resolved on the white matter skeleton. The fornix is largely implicated in memory formation,25,26 whereas the stria terminalis parallels the fornix and is believed to be important in emotional processing, mood, and anxiety.27,28 Aside from alterations in visual memory, there were no clinical findings in our study to suggest changes in emotional processing, though it is possible that the lack of detectable changes may be due to the subtle, subclinical nature of white matter injury during a limited period of a single football season.
There are limitations to our study. First, the sample size was small and did not include a group of nonexposed, age-matched controls; therefore, it is not possible to control for alterations in white matter fractional anisotropy related to brain maturation during the season. Second, data suggest that the reproducibility of the ENIGMA-DTI protocol (http://enigma.usc.edu/protocols/dti-protocols/) overall is excellent, though the fornix is a region where there is a greater degree of variability among imaging studies.29 It is therefore possible that the observed changes in the FXST are due to type I error in our small sample and are not generalizable; however, despite these factors, the finding remained robust to correction for multiple comparisons in the context of our mixed linear model. Nevertheless, we consider this pilot study exploratory and hypothesis-generating, necessitating confirmation in a larger sample. Third, given the small sample size, heterogeneity of trauma, and subtle alterations in white matter FA, type II error is a possibility as well. Perhaps for this reason, voxelwise analysis, with appropriate corrections for multiple comparisons, failed to detect relationships found with the ROI analysis. We believe that using an ROI approach in conjunction with the Tract-Based Spatial Statistics analysis increased sensitivity to subtle changes in white matter without relying on perfect registration, as would be the case in a voxelwise comparison. Moreover, the mixed model allowed a more flexible statistical approach, incorporating a random variable for subject, recognizing that response to head impacts might differ across individuals as a function of a variety of genetic, physiologic, and/or environmental factors.
It is unclear whether most of the measured changes during a single football season might be reversible with rest. Our data in a single subject who sustained a concussion during the season showed an approximately 5% decrease in FXST FA within 24 hours after the concussion, but these values normalized at the postseason scan after several weeks of rest. Although anecdotal, this evidence confirms the need in future studies for imaging follow-up after sustained periods of rest from contact exposure.
Conclusions
This pilot study revealed subtle decreases in fractional anisotropy within the white matter tracts of the limbic system that worsened with an increasing number of accelerometer-measured impacts during the season. The white matter changes observed on imaging in the FXST correlated with declines in visual memory, but not other neurocognitive measures. These findings suggest the possibility of white matter injury due to repetitive subclinical impacts while participating in a single season of high school football; however, they will require validation with appropriately powered, hypothesis-driven studies including age-matched controls. Longitudinal studies spanning multiple sequential football seasons with a cohort of subjects will also be useful to evaluate the effects of cumulative trauma, normalization of white matter alterations after rest, and the presence of white matter changes too subtle to manifest in the short period of 1 football season.
Footnotes
Disclosures: Michael D. Clark—RELATED: Grant: National Institute of Neurological Disorders and Stroke, Comments: grant No. F30NS090816.* *Money paid to the institution.
This work was supported by Department Seed Funding, Department of Radiology, Duke University, Durham, North Carolina, and the National Institutes of Health under the Ruth L. Kirschstein National Research Service Award F30NS090816 from the National Institute of Neurological Disorders and Stroke.
Paper previously presented, in part, as a scientific poster at: Annual Meeting of the Radiological Society of North America, November 30–December 5, 2014; Chicago, Illinois.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received June 11, 2017.
- Accepted after revision October 3, 2017.
- © 2018 by American Journal of Neuroradiology